
How many $\pi $ bonds are there in $C{{O}_{2}}$?
Answer
558.6k+ views
Hint To solve this question, first we have to draw the structure of carbon dioxide i.e. $C{{O}_{2}}$. We should know the difference between sigma bond and pi bond.
Complete step by step solution:
$C{{O}_{2}}$ means carbon dioxide. C is the chemical symbol for carbon and O is the chemical symbol for oxygen. And there are two oxygen present with carbon that’s why the suffix di is used.
We know that,
The atomic number of carbon = 6
The number of valence electrons in carbon = 4
The atomic number of oxygen = 8
The number of valence electrons in oxygen = 6
The structure of carbon dioxide is mentioned below:
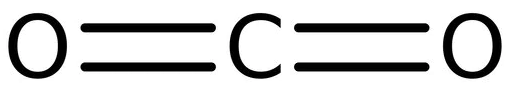
Carbon dioxide contains two double bonds and we know that each double bond consists of one sigma and one pi bond.
Hence, 2 $\pi $ bonds are there in $C{{O}_{2}}$.
Additional information:
The chemical symbol is used to represent an atom or an element. The chemical symbol of an element usually consists of one or two letters from the Latin alphabet and the first letter is always capital. There are many sources from which the symbols are derived, it includes Greek name of an element or German name etc. For example: the symbol for lead is Pb and it is derived from the Latin name of lead with is plumbum, the symbol for tungsten is W and it is derived from the German name of tungsten which is Wolfram etc.
Note: The sigma bonds are formed by the axial overlapping of half-filled atomic orbitals of the atoms whereas pi bonds are formed by the lateral overlapping of the half-filled atomic orbitals.
Complete step by step solution:
$C{{O}_{2}}$ means carbon dioxide. C is the chemical symbol for carbon and O is the chemical symbol for oxygen. And there are two oxygen present with carbon that’s why the suffix di is used.
We know that,
The atomic number of carbon = 6
The number of valence electrons in carbon = 4
The atomic number of oxygen = 8
The number of valence electrons in oxygen = 6
The structure of carbon dioxide is mentioned below:
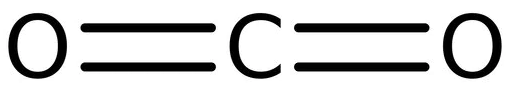
Carbon dioxide contains two double bonds and we know that each double bond consists of one sigma and one pi bond.
Hence, 2 $\pi $ bonds are there in $C{{O}_{2}}$.
Additional information:
The chemical symbol is used to represent an atom or an element. The chemical symbol of an element usually consists of one or two letters from the Latin alphabet and the first letter is always capital. There are many sources from which the symbols are derived, it includes Greek name of an element or German name etc. For example: the symbol for lead is Pb and it is derived from the Latin name of lead with is plumbum, the symbol for tungsten is W and it is derived from the German name of tungsten which is Wolfram etc.
Note: The sigma bonds are formed by the axial overlapping of half-filled atomic orbitals of the atoms whereas pi bonds are formed by the lateral overlapping of the half-filled atomic orbitals.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




