
How will you convert phenol to benzene?
Answer
526k+ views
Hint: Benzene in the laboratory is prepared from the process which involves the reduction of Phenol. The method is commercial and so chosen as a suitable procedure to produce Benzene.
Complete answer:
First, let us look at the structures of benzene and phenol. Benzene is a six membered ring with alternating double and single bonds; each carbon atom has one hydrogen atom attached to it. Phenol has a structure similar to benzene except one of the hydrogen atoms attached to carbon is replaced by a hydroxyl group. The skeleton structures are as follows:
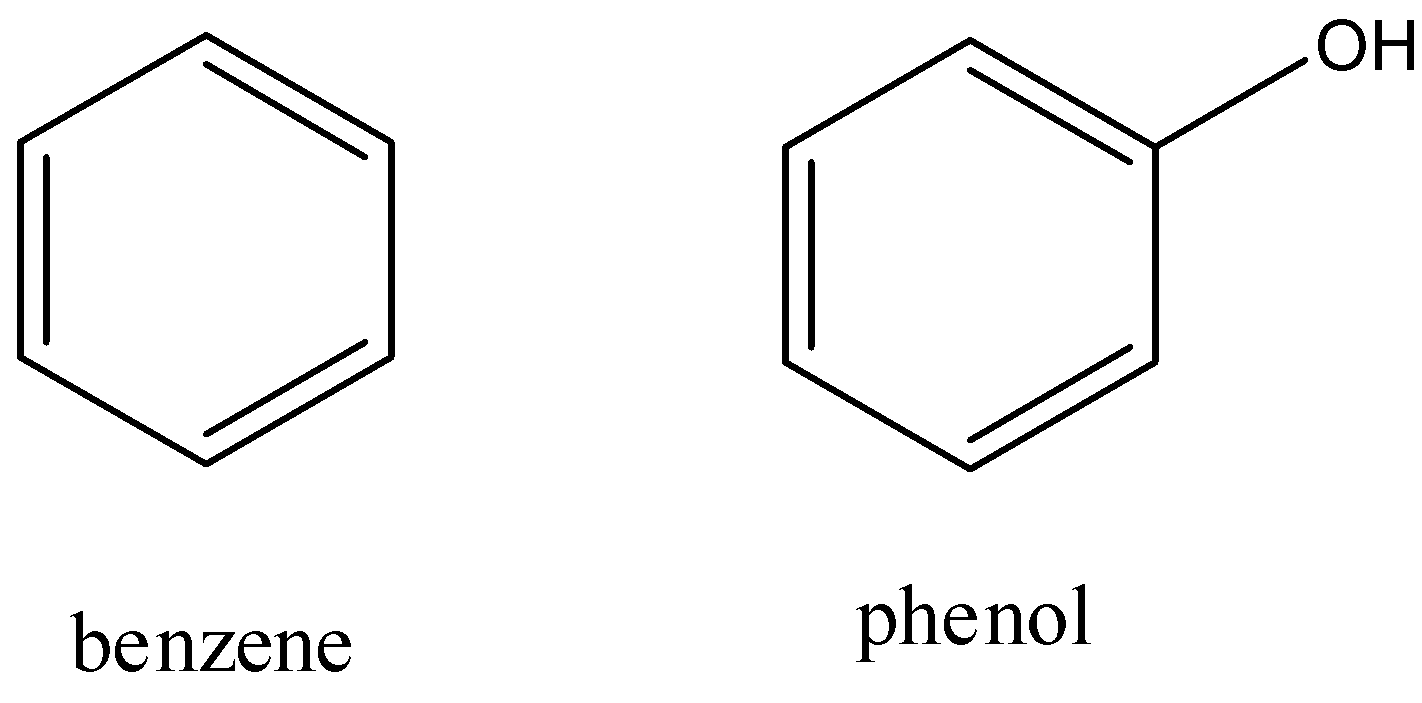
Now, to form benzene from phenol, the commonly used industrial method is to reduce benzene using red hot zinc powder and distillation. The reaction is as follows:
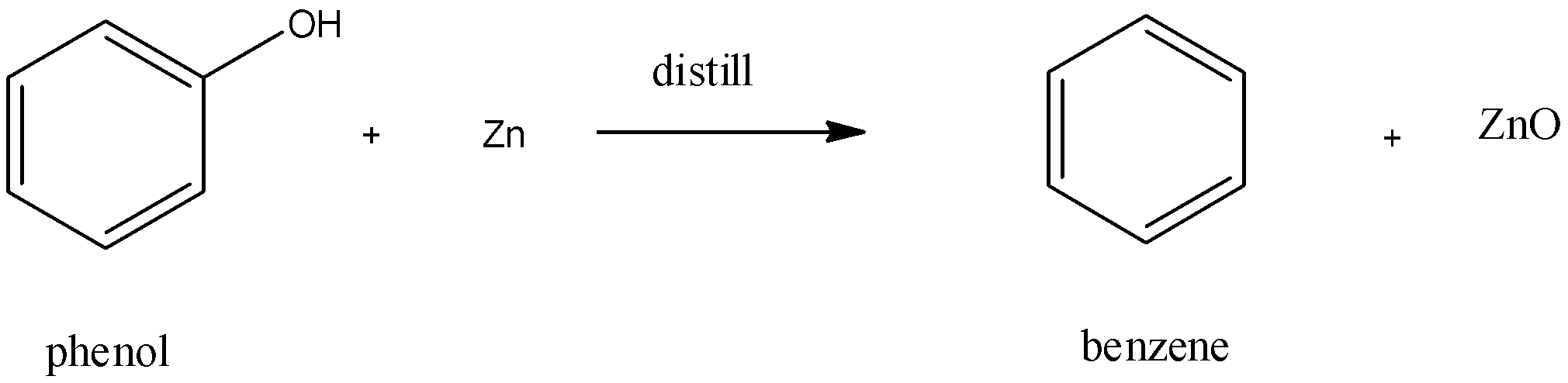
($-OH$ group of the benzene nucleus is replaced by $-H$ in this reaction)
The above figure shows the conversion of phenol into benzene. Phenol gives out proton and forms phenoxy anion. This anion later gives out oxygen radical to form phenyl - free radical. On the other hand, zinc reacts with the oxygen radicals to form Zinc Oxide (ZnO). Finally, hydrogen free radical (formed from hydrogen ion) joins with phenyl - free radical to form Benzene.
Note:
The molecular formula of benzene is ${{C}_{6}}{{H}_{6}}$ this indicates that it is a highly unsaturated compound and is thus unstable. But a lot of stability is seen when benzene reacts with other substances; this happens due to a phenomenon called resonance. The resonance keeps the benzene ring stable. The formation of free radicals in this reaction will require a lot of energy, so the zinc has to be red hot so that the reaction can move forward.
Complete answer:
First, let us look at the structures of benzene and phenol. Benzene is a six membered ring with alternating double and single bonds; each carbon atom has one hydrogen atom attached to it. Phenol has a structure similar to benzene except one of the hydrogen atoms attached to carbon is replaced by a hydroxyl group. The skeleton structures are as follows:

Now, to form benzene from phenol, the commonly used industrial method is to reduce benzene using red hot zinc powder and distillation. The reaction is as follows:

($-OH$ group of the benzene nucleus is replaced by $-H$ in this reaction)
The above figure shows the conversion of phenol into benzene. Phenol gives out proton and forms phenoxy anion. This anion later gives out oxygen radical to form phenyl - free radical. On the other hand, zinc reacts with the oxygen radicals to form Zinc Oxide (ZnO). Finally, hydrogen free radical (formed from hydrogen ion) joins with phenyl - free radical to form Benzene.
Note:
The molecular formula of benzene is ${{C}_{6}}{{H}_{6}}$ this indicates that it is a highly unsaturated compound and is thus unstable. But a lot of stability is seen when benzene reacts with other substances; this happens due to a phenomenon called resonance. The resonance keeps the benzene ring stable. The formation of free radicals in this reaction will require a lot of energy, so the zinc has to be red hot so that the reaction can move forward.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




