
Hybridisation of carbon in ${C_3}{O_2}$ is:
(A) sp
(B) $s{p^2}$
(C) $s{p^3}$
(D) $s{p^3}d$
Answer
589.5k+ views
Hint: First see the number of electrons in the outermost orbits of the element and then count the number of bonding regions and lone pairs formed by it in the particular compound. Accordingly see what the hybridisation will be.
Complete answer:
-Hybridisation is a basic concept of mixing of atomic orbitals to form new hybrid orbitals to form chemical bonds according to the valence bond theory.
-Before trying to deduce the hybridisation let us check a little about carbon atoms. It has an atomic number of 6 and its electronic configuration is: $1{s^2}2{s^2}2{p^2}$. We know that it can accommodate 4 more electrons in its outermost shell and form a maximum of 4 bonds.
-${C_3}{O_2}$ is the molecular formula of carbon suboxide or tricarbon dioxide. It is an oxide of carbon and also a member of the linear oxocarbon series ($O = {C_n} = O$).
Its structure is as follows:
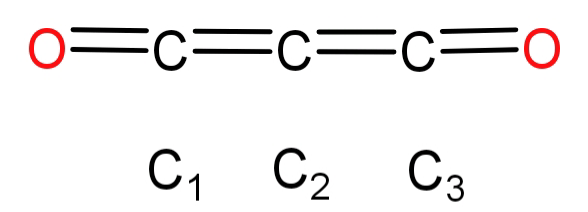
In this we can see that each carbon forms double bonds with 2 other atoms (either other carbon or oxygen) and each carbon has 2 sigma (σ) and 2 pi (π) bonds. Also carbon has 4 electrons in its outermost shell and it cannot form more than 4 bonds, so no more bonding regions are possible. Let us just see the 3 carbon atoms and their bonding patterns:
For carbon 1 (${C_1}$): We can see that it forms 1 double bond with an oxygen atom and one with another carbon atom and hence has only 2 bonding domains. It forms 2 sigma (σ) and 2 pi (π) bonds. Hence its hybridisation is sp.
For carbon 2 (${C_2}$): We can see that it forms 2 double bonds, each with a different carbon atom and hence has only 2 bonding domains. It forms 2 sigma (σ) and 2 pi (π) bonds. Hence its hybridisation is sp.
For carbon 3 (${C_3}$):We can see that it forms 1 double bond with an oxygen atom and one with another carbon atom and hence has only 2 bonding domains. It forms 2 sigma (σ) and 2 pi (π) bonds. Hence its hybridisation is sp.
Hence we can say that the hybridisation of carbon in ${C_3}{O_2}$ is sp.
The correct option will be: (A) sp
Note:
Remember that a double bond is counted as 1 electron region only and not 2 electron regions. Carbon superoxide is also considered as anhydride of malonic anhydride or second anhydride of malonic acid because it can be formed by heating a dry mixture of malonic acid (or its ester) and phosphorous pentoxide.
Complete answer:
-Hybridisation is a basic concept of mixing of atomic orbitals to form new hybrid orbitals to form chemical bonds according to the valence bond theory.
-Before trying to deduce the hybridisation let us check a little about carbon atoms. It has an atomic number of 6 and its electronic configuration is: $1{s^2}2{s^2}2{p^2}$. We know that it can accommodate 4 more electrons in its outermost shell and form a maximum of 4 bonds.
-${C_3}{O_2}$ is the molecular formula of carbon suboxide or tricarbon dioxide. It is an oxide of carbon and also a member of the linear oxocarbon series ($O = {C_n} = O$).
Its structure is as follows:

In this we can see that each carbon forms double bonds with 2 other atoms (either other carbon or oxygen) and each carbon has 2 sigma (σ) and 2 pi (π) bonds. Also carbon has 4 electrons in its outermost shell and it cannot form more than 4 bonds, so no more bonding regions are possible. Let us just see the 3 carbon atoms and their bonding patterns:
For carbon 1 (${C_1}$): We can see that it forms 1 double bond with an oxygen atom and one with another carbon atom and hence has only 2 bonding domains. It forms 2 sigma (σ) and 2 pi (π) bonds. Hence its hybridisation is sp.
For carbon 2 (${C_2}$): We can see that it forms 2 double bonds, each with a different carbon atom and hence has only 2 bonding domains. It forms 2 sigma (σ) and 2 pi (π) bonds. Hence its hybridisation is sp.
For carbon 3 (${C_3}$):We can see that it forms 1 double bond with an oxygen atom and one with another carbon atom and hence has only 2 bonding domains. It forms 2 sigma (σ) and 2 pi (π) bonds. Hence its hybridisation is sp.
Hence we can say that the hybridisation of carbon in ${C_3}{O_2}$ is sp.
The correct option will be: (A) sp
Note:
Remember that a double bond is counted as 1 electron region only and not 2 electron regions. Carbon superoxide is also considered as anhydride of malonic anhydride or second anhydride of malonic acid because it can be formed by heating a dry mixture of malonic acid (or its ester) and phosphorous pentoxide.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




