
Hybridization in ${\text{S}}{{\text{O}}_2}$ molecule is:
A. ${\text{sp}}$
B. ${\text{s}}{{\text{p}}^2}$
C. ${\text{s}}{{\text{p}}^3}$
D. ${\text{s}}{{\text{p}}^3}{\text{d}}$
Answer
585.9k+ views
Hint: Covalent compounds are formed when the electrons are shared to complete the octet. Sulfur and oxygen are non-metals. They have very high electronegativity. Thus they form a covalent bond. While an ionic bond is formed between metal and non-metal.
Complete step by step answer: There are four types of chemical bonding-ionic, covalent, hydrogen and metallic bonding. Covalent bonding is generally formed between two nonmetals. It is a type of chemical bond in which there is mutual sharing of electrons between two atoms. It is further classified into single, double and triple covalent bonds with respect to mutual sharing of one, two and three bonds respectively.
Consider ${\text{S}}{{\text{O}}_2}$ molecule in which sulfur has six electrons in a valence shell. So to form an octet, it is easy to gain two electrons than lose six electrons. Therefore its valency is two. Similarly, the valency of oxygen is two. The electronic configuration of sulfur is $1{{\text{s}}^2}2{{\text{s}}^2}2{{\text{p}}^6}3{s^2}3{{\text{p}}^4}$. First two shells are completely filled. There are two electrons in $3{\text{s}}$ orbital four electrons in $3{\text{p}}$ orbital and it needs four unpaired electrons. One electron in $3{{\text{p}}_{\text{x}}}$ jumps to $3{\text{d}}$ orbital. This leads to formation of one unpaired electron in $3{\text{d}}$ orbital and three unpaired electrons in $3{\text{p}}$ orbital. Two $3{\text{p}}$ orbitals and one $3{\text{s}}$ orbital gets hybridized, thereby forming three ${\text{s}}{{\text{p}}^2}$ hybrid orbitals. Two hybrid orbitals form $\sigma $ bonds with oxygen. While $3{\text{d}}$ and $3{\text{p}}$ orbitals are involved in the formation of $\pi $ bonds. Moreover, the hybridization of oxygen is also ${\text{s}}{{\text{p}}^2}$ . Therefore it forms ${\text{s}}{{\text{p}}^2}$ hybridization.
Hence the correct option is B.
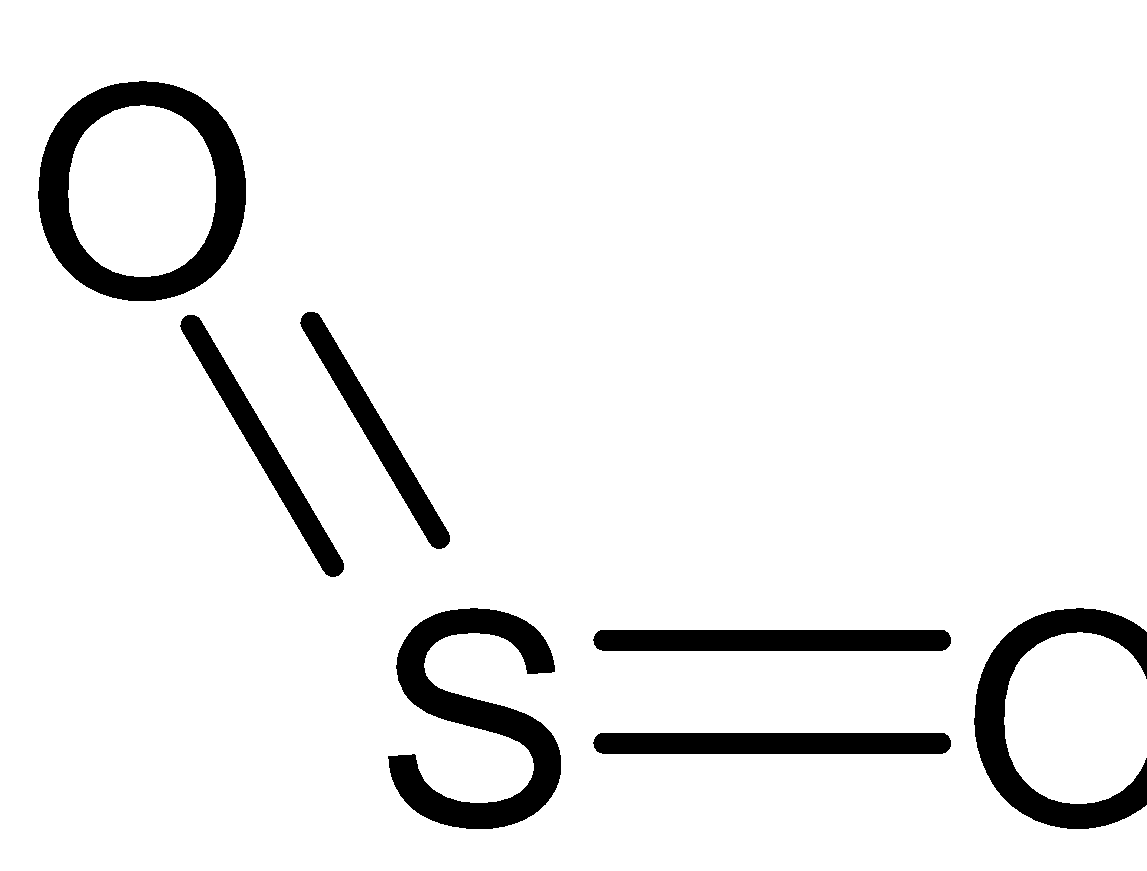
Note: The molecular geometry of ${\text{S}}{{\text{O}}_2}$ is V-shaped or bent and the bond angle is ${119^ \circ }$. Two double bonds are there and a lone pair of electrons is there in one hybrid orbital, thereby forming bent shape. The structure of ${\text{S}}{{\text{O}}_2}$ molecule is given below:
Complete step by step answer: There are four types of chemical bonding-ionic, covalent, hydrogen and metallic bonding. Covalent bonding is generally formed between two nonmetals. It is a type of chemical bond in which there is mutual sharing of electrons between two atoms. It is further classified into single, double and triple covalent bonds with respect to mutual sharing of one, two and three bonds respectively.
Consider ${\text{S}}{{\text{O}}_2}$ molecule in which sulfur has six electrons in a valence shell. So to form an octet, it is easy to gain two electrons than lose six electrons. Therefore its valency is two. Similarly, the valency of oxygen is two. The electronic configuration of sulfur is $1{{\text{s}}^2}2{{\text{s}}^2}2{{\text{p}}^6}3{s^2}3{{\text{p}}^4}$. First two shells are completely filled. There are two electrons in $3{\text{s}}$ orbital four electrons in $3{\text{p}}$ orbital and it needs four unpaired electrons. One electron in $3{{\text{p}}_{\text{x}}}$ jumps to $3{\text{d}}$ orbital. This leads to formation of one unpaired electron in $3{\text{d}}$ orbital and three unpaired electrons in $3{\text{p}}$ orbital. Two $3{\text{p}}$ orbitals and one $3{\text{s}}$ orbital gets hybridized, thereby forming three ${\text{s}}{{\text{p}}^2}$ hybrid orbitals. Two hybrid orbitals form $\sigma $ bonds with oxygen. While $3{\text{d}}$ and $3{\text{p}}$ orbitals are involved in the formation of $\pi $ bonds. Moreover, the hybridization of oxygen is also ${\text{s}}{{\text{p}}^2}$ . Therefore it forms ${\text{s}}{{\text{p}}^2}$ hybridization.
Hence the correct option is B.
Note: The molecular geometry of ${\text{S}}{{\text{O}}_2}$ is V-shaped or bent and the bond angle is ${119^ \circ }$. Two double bonds are there and a lone pair of electrons is there in one hybrid orbital, thereby forming bent shape. The structure of ${\text{S}}{{\text{O}}_2}$ molecule is given below:

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




