
What is the hybridization state of B and N in inorganic benzene respectively?
(A) \[s{p^2} and {\rm{ }} s{p^3}\]
(B) \[s{p^3} and {\rm{ }} s{p^2}\]
(C) \[Both{\rm{ }}\,s{p^2}\]
(D) \[Both{\rm{ }}\,s{p^3}\]
Answer
571.8k+ views
Hint: Borazine is called as the inorganic benzene, this is because borazine resembles benzene in the structure and few properties. If we know the hybridisation of the carbon atom in benzene, it would be easy for us to compare.
Complete answer:
Before we directly move into the hybridisation state of $B$ and $N$ in inorganic benzene, we must know what a borazine is. Borazine is a chemical compound with the molecular formula of \[{B_3}{N_3}{H_3}\]. Borazine is a colourless liquid. It has an aromatic smell. It was first prepared from the chemical reaction of diborane and ammonia in the year 1926. It was called inorganic benzene because it is isoelectronic with benzene. Borazine is having a structure which is similar to the benzene, i.e. planar hexagonal structure. In borazine structure, Boron and Nitrogen atoms are arranged alternatively.
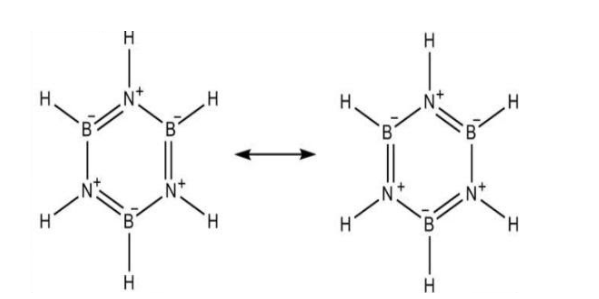
In the borazine structure, Boron is bonded to three atoms one hydrogen and two Nitrogen atoms. As it is bonded to three atoms, each boron atom in borazine will be having \[s{p^2}\] hybridisation and trigonal planar structure.
The nitrogen atom in borazine is bonded to three atoms namely two boron atom and one hydrogen atom. Like boron, as nitrogen is also bonded to three atoms, the hybridization will also be \[s{p^2}\].
Therefore, both B and N in inorganic benzene i.e. borazine will be having \[s{p^2}\] hybridisation.
Hence the correct answer will be option (C) \[Both{\rm{ }}s{p^2}\].
Additional information:
Difference between benzene and borazine
Note: As we know that benzene and borazine are having similar structure, i.e. hexagonal structure. In benzene, the carbon atom is having \[s{p^2}\] hybridisation. Therefore, like benzene, in borazine, N and B should also have \[s{p^2}\] hybridisation.
Complete answer:
Before we directly move into the hybridisation state of $B$ and $N$ in inorganic benzene, we must know what a borazine is. Borazine is a chemical compound with the molecular formula of \[{B_3}{N_3}{H_3}\]. Borazine is a colourless liquid. It has an aromatic smell. It was first prepared from the chemical reaction of diborane and ammonia in the year 1926. It was called inorganic benzene because it is isoelectronic with benzene. Borazine is having a structure which is similar to the benzene, i.e. planar hexagonal structure. In borazine structure, Boron and Nitrogen atoms are arranged alternatively.

In the borazine structure, Boron is bonded to three atoms one hydrogen and two Nitrogen atoms. As it is bonded to three atoms, each boron atom in borazine will be having \[s{p^2}\] hybridisation and trigonal planar structure.
The nitrogen atom in borazine is bonded to three atoms namely two boron atom and one hydrogen atom. Like boron, as nitrogen is also bonded to three atoms, the hybridization will also be \[s{p^2}\].
Therefore, both B and N in inorganic benzene i.e. borazine will be having \[s{p^2}\] hybridisation.
Hence the correct answer will be option (C) \[Both{\rm{ }}s{p^2}\].
Additional information:
Difference between benzene and borazine
| BORAZINE | BENZENE |
| Molecular formula- \[{B_3}{N_3}{H_3}\] | Molecular formula - \[{C_6}{H_6}\] |
| Inorganic compound | Organic compound |
| Contains B and N | Contains only C |
| Reactive | Less reactive |
| Not a perfect hexagon | perfect hexagon |
Note: As we know that benzene and borazine are having similar structure, i.e. hexagonal structure. In benzene, the carbon atom is having \[s{p^2}\] hybridisation. Therefore, like benzene, in borazine, N and B should also have \[s{p^2}\] hybridisation.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




