
Hydrogen being a highly inflammable gas and oxygen being a supporter of combustion, yet water which is a compound made up of hydrogen and oxygen is used to extinguish fire. Why?
Answer
520.8k+ views
Hint :Elements are substances which contain only one type of atom in their structure. They are pure and are arranged in periodic tables to make their study easier. Compounds are formed when a fixed ratio of two or more than two elements are combined chemically to form bonds which keep them together.
Complete Step By Step Answer:
Hydrogen which is an element has its own properties due to the atoms it has like hydrogen is highly inflammable. This is the property of hydrogen atoms. Oxygen is also an element which has its own properties, which are governed by its atoms. As oxygen atoms have the property to support combustion reactions.
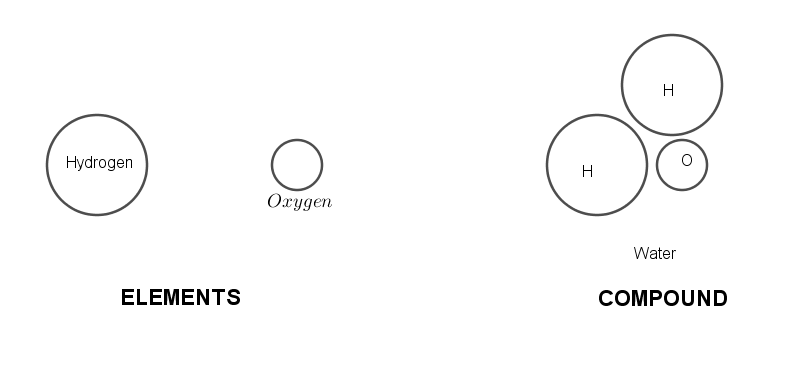
Whereas, a water is a compound, and compounds have properties different from its constituents and the constituents cannot retain their own properties when combined. Thus water which is made up of hydrogen and oxygen in a fixed ratio of $ 1:8 $ by mass, has properties which are very different from its elements. Water is used as a fire extinguisher whereas both of its elements have very contrast properties.
$ 2{H_2} + {O_2} \to 2{H_2}O $
Water is generally present as liquid in state at room temperature whereas hydrogen and oxygen both are gases. Thus physical properties of compounds also differ from the elements.
Note :
Water present in rivers or oceans the structure of water remains always the same and the ratio with which the elements combine to form the compounds also does not change.
Complete Step By Step Answer:
Hydrogen which is an element has its own properties due to the atoms it has like hydrogen is highly inflammable. This is the property of hydrogen atoms. Oxygen is also an element which has its own properties, which are governed by its atoms. As oxygen atoms have the property to support combustion reactions.

Whereas, a water is a compound, and compounds have properties different from its constituents and the constituents cannot retain their own properties when combined. Thus water which is made up of hydrogen and oxygen in a fixed ratio of $ 1:8 $ by mass, has properties which are very different from its elements. Water is used as a fire extinguisher whereas both of its elements have very contrast properties.
$ 2{H_2} + {O_2} \to 2{H_2}O $
Water is generally present as liquid in state at room temperature whereas hydrogen and oxygen both are gases. Thus physical properties of compounds also differ from the elements.
Note :
Water present in rivers or oceans the structure of water remains always the same and the ratio with which the elements combine to form the compounds also does not change.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




