
When hydrogen peroxide is treated with a cold acidified \[{K_2}C{r_2}{O_7}\] solution containing ether, blue colour is obtained. This is due to:
A. Perchromic acid
B. Potassium chromate
C. Chromium sulphate
D. Chromium trioxide
Answer
573.6k+ views
Hint:Potassium dichromate or \[{K_2}C{r_2}{O_7}\] is an orange coloured oxidizing agent, used in various reactions. On the other hand, hydrogen peroxide or \[{H_2}{O_2}\] is a pale blue viscous liquid. They react with each other and give blue colour compounds.
Complete answer:
As we know, potassium dichromate \[\left( {{K_2}C{r_2}{O_7}} \right)\] is orange in colour and on reaction with pale blue hydrogen peroxide, it yields blue colour compound. To identify the blue colour compound we must write the chemical reaction as follows:
\[{K_2}C{r_2}{O_7} + 4{H_2}{O_2} + {H_2}S{O_4}\xrightarrow{{{\text{Ether}}}}Cr{O_5} + {K_2}S{O_4} + 5{H_2}O\]
The reaction proceeds in three different steps:
Step I: \[{K_2}C{r_2}{O_7} + {H_2}S{O_4} \to {K_2}S{O_4} + {H_2}C{r_2}{O_7}\]
Step II: \[4{H_2}{O_2} \to 4{H_2}O + 4\left( O \right)\]
Step III: \[{H_2}C{r_2}{O_7} + 4\left( O \right) \to 2Cr{O_5} + {H_2}O\]
Hence the three products formed are \[Cr{O_5}\], \[{K_2}S{O_4}\] and \[{H_2}O\]. Among these, \[Cr{O_5}\] or chromium pentoxide has a blue colour in ether.
In the above reaction, the oxidation state of hydrogen peroxide changes from \[ - 1\] to \[ - 2\] as it acts as an oxidizing agent in the reaction.
In less acidic conditions, reaction of hydrogen peroxide with potassium dichromate gives a deep blue-violet coloured compound. Whereas, in an alkaline solution this reaction gives a red-brown coloured compound.
Hence the correct option is A, per-chromic acid.
Additional information:
\[Cr{O_5}\] or chromium pentoxide is also known as perchromic acid and it has a structure of butterfly.
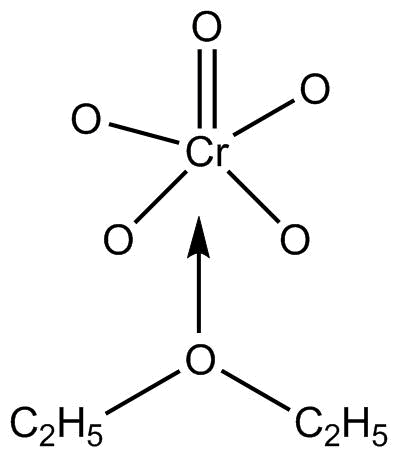
The structure is so, due to two peroxy linkages in the compound.
Note:
Potassium dichromate is an oxidizing agent that oxides aldehydes and alcohols (primary and secondary). It is toxic in nature and also known to be a carcinogen and a mutagen. Therefore, it can be fatal for the environment. It is also a common reason behind dermatitis, especially on arms and hands.
Complete answer:
As we know, potassium dichromate \[\left( {{K_2}C{r_2}{O_7}} \right)\] is orange in colour and on reaction with pale blue hydrogen peroxide, it yields blue colour compound. To identify the blue colour compound we must write the chemical reaction as follows:
\[{K_2}C{r_2}{O_7} + 4{H_2}{O_2} + {H_2}S{O_4}\xrightarrow{{{\text{Ether}}}}Cr{O_5} + {K_2}S{O_4} + 5{H_2}O\]
The reaction proceeds in three different steps:
Step I: \[{K_2}C{r_2}{O_7} + {H_2}S{O_4} \to {K_2}S{O_4} + {H_2}C{r_2}{O_7}\]
Step II: \[4{H_2}{O_2} \to 4{H_2}O + 4\left( O \right)\]
Step III: \[{H_2}C{r_2}{O_7} + 4\left( O \right) \to 2Cr{O_5} + {H_2}O\]
Hence the three products formed are \[Cr{O_5}\], \[{K_2}S{O_4}\] and \[{H_2}O\]. Among these, \[Cr{O_5}\] or chromium pentoxide has a blue colour in ether.
In the above reaction, the oxidation state of hydrogen peroxide changes from \[ - 1\] to \[ - 2\] as it acts as an oxidizing agent in the reaction.
In less acidic conditions, reaction of hydrogen peroxide with potassium dichromate gives a deep blue-violet coloured compound. Whereas, in an alkaline solution this reaction gives a red-brown coloured compound.
Hence the correct option is A, per-chromic acid.
Additional information:
\[Cr{O_5}\] or chromium pentoxide is also known as perchromic acid and it has a structure of butterfly.

The structure is so, due to two peroxy linkages in the compound.
Note:
Potassium dichromate is an oxidizing agent that oxides aldehydes and alcohols (primary and secondary). It is toxic in nature and also known to be a carcinogen and a mutagen. Therefore, it can be fatal for the environment. It is also a common reason behind dermatitis, especially on arms and hands.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




