
What is the hydrogenated product of triolein?
Answer
553.8k+ views
Hint: We know that hydrogenation is the process of reaction between molecular hydrogen with another compound or element usually in the presence of a catalyst such as nickel, palladium, or platinum under normal conditions of temperature and pressure and hydrogen gets added up.
Complete step by step answer:
Triolein is triacylglycerol with three oleic acid groups that undergo hydrogenation in presence of a palladium catalyst which is the addition of molecular hydrogen to the unsaturated sites and converting it into saturated sites.
The unsaturated double bonds are converted to saturated single bonds with the addition of molecular hydrogen. It forms a triacylglycerol consisting of stearic acid groups.
The hydrogen here adds to all the double bonds of the glyceryl trioleate (triolein) using a palladium catalyst and forms glyceryl tristearate that is tristearin
The following reaction takes place in the following condition
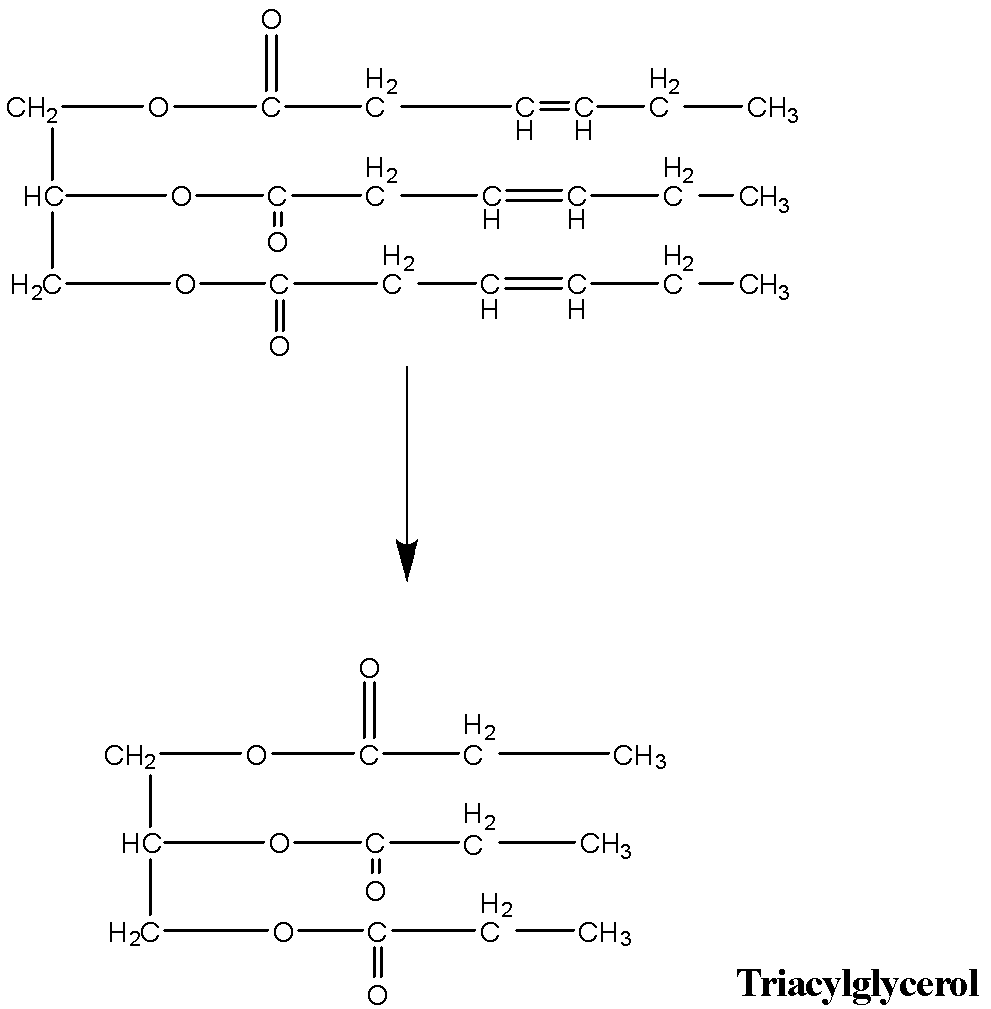
Therefore the hydrogenated product of triolein is glyceryl tristearate that is tristearin.
Note: In the process of hydrogenation, the most commonly used reagents are metals such as nickel, palladium, and platinum and their oxides. Also for high-pressure hydrogenation reaction copper chromite and nickel with the support of kieselguhr are mainly used.
Hydrogenation reduces the number of double bonds and triple bonds in hydrocarbons.
Triolein is a triglyceride that is symmetrical derived from glycerol and three units of unsaturated fatty acid oleic acid.
Hydrogenation is not thermodynamically favored at normal temperature so a catalyst is needed and that catalyst is usually a metal.
Triolein is also known as glyceryl trioleate and is one of the two components of Lorenzo’s oil.
Complete step by step answer:
Triolein is triacylglycerol with three oleic acid groups that undergo hydrogenation in presence of a palladium catalyst which is the addition of molecular hydrogen to the unsaturated sites and converting it into saturated sites.
The unsaturated double bonds are converted to saturated single bonds with the addition of molecular hydrogen. It forms a triacylglycerol consisting of stearic acid groups.
The hydrogen here adds to all the double bonds of the glyceryl trioleate (triolein) using a palladium catalyst and forms glyceryl tristearate that is tristearin
The following reaction takes place in the following condition

Therefore the hydrogenated product of triolein is glyceryl tristearate that is tristearin.
Note: In the process of hydrogenation, the most commonly used reagents are metals such as nickel, palladium, and platinum and their oxides. Also for high-pressure hydrogenation reaction copper chromite and nickel with the support of kieselguhr are mainly used.
Hydrogenation reduces the number of double bonds and triple bonds in hydrocarbons.
Triolein is a triglyceride that is symmetrical derived from glycerol and three units of unsaturated fatty acid oleic acid.
Hydrogenation is not thermodynamically favored at normal temperature so a catalyst is needed and that catalyst is usually a metal.
Triolein is also known as glyceryl trioleate and is one of the two components of Lorenzo’s oil.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




