
How do hydrophobic amino acids and hydrophilic amino acids cause proteins to have a specific shape?
Answer
541.8k+ views
Hint :For solving this question first, we need to know the exact meaning of hydrophobic amino acids and hydrophilic amino acids. Amino acids are classified on the basis of their side chains. Hydrophobic side chains are composed of carbon and hydrogen and have very small dipole moments, and they tend to be repelled from water. The nine amino acids that are hydrophobic in nature are glycine, alanine, valine, leucine, isoleucine, proline, phenylalanine, methionine, and tryptophan. Hydrophilic, or which is also called as water-soluble amino acids, have ionized or polar side chains.
Complete Step By Step Answer:
Proteins are made up of amino acids which are used for different purposes in the cell. The cell is an aqueous environment. The hydrophilic amino acids interact more strongly with water than the hydrophobic amino acids. The interactions of the amino acids within the aqueous environment of the cell results in a specific protein shape.
Some examples of hydrophobic amino acids are valine, isoleucine, leucine, methionine.
Some examples of hydrophilic amino acids are lysine, arginine, histidine.
Structure of valine
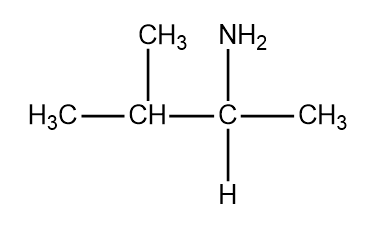
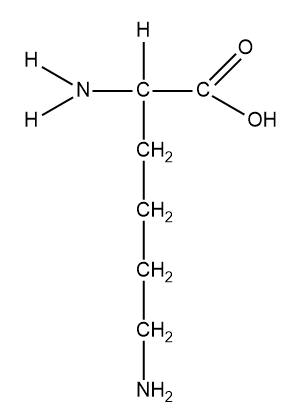
Structure of lysine
The four types of protein structure are primary, secondary, and tertiary.
Note :
There are four structural levels in proteins:
a. Primary structure - The amino acids are linked with each other by peptide bonds only.
b. Secondary structure: Long polypeptide chains fold or coil together to form secondary structure. These secondary structures are produced and maintained by hydrogen bonding, Two types of secondary structures are: α helix and β pleated sheets
c. Tertiary structure: The polypeptide chain may undergo coiling and folding to produce the tertiary structure. These structures are stabilised by the several types of bonds namely hydrogen bond, ionic bond, van der waals interaction, covalent bond (disulphide bridges) and hydrophobic bond.
d. Quaternary structure: Protein is said to be in quaternary structure if it consists of two or more polypeptide chains united by forces other than covalent bond. The forces which stabilise these structures are hydrogen bond and electrostatic bond.
Complete Step By Step Answer:
Proteins are made up of amino acids which are used for different purposes in the cell. The cell is an aqueous environment. The hydrophilic amino acids interact more strongly with water than the hydrophobic amino acids. The interactions of the amino acids within the aqueous environment of the cell results in a specific protein shape.
Some examples of hydrophobic amino acids are valine, isoleucine, leucine, methionine.
Some examples of hydrophilic amino acids are lysine, arginine, histidine.
Structure of valine

Structure of lysine

The four types of protein structure are primary, secondary, and tertiary.
Note :
There are four structural levels in proteins:
a. Primary structure - The amino acids are linked with each other by peptide bonds only.
b. Secondary structure: Long polypeptide chains fold or coil together to form secondary structure. These secondary structures are produced and maintained by hydrogen bonding, Two types of secondary structures are: α helix and β pleated sheets
c. Tertiary structure: The polypeptide chain may undergo coiling and folding to produce the tertiary structure. These structures are stabilised by the several types of bonds namely hydrogen bond, ionic bond, van der waals interaction, covalent bond (disulphide bridges) and hydrophobic bond.
d. Quaternary structure: Protein is said to be in quaternary structure if it consists of two or more polypeptide chains united by forces other than covalent bond. The forces which stabilise these structures are hydrogen bond and electrostatic bond.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




