
Identify the product in the following reaction.
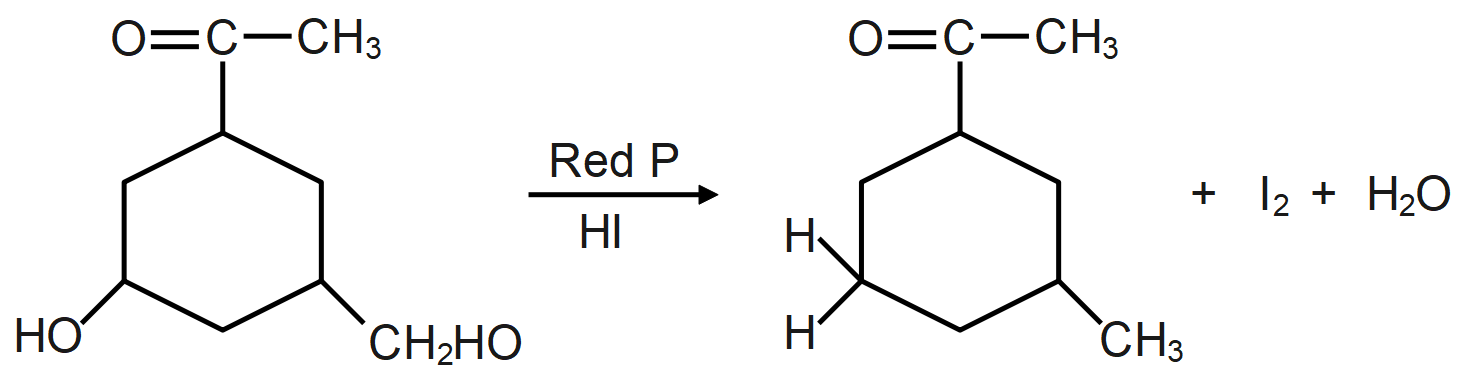

Answer
497.7k+ views
Hint: We know that the red phosphorus and HI are used in reduction. Hence, they will help in addition of hydrogen to the substrate molecule. Reduction is defined as the addition of hydrogen or removal of oxygen from the substrate molecule.
Complete Step By Step Answer:
As we know, the reaction of a given compound with red phosphorus and HI results in the formation of alkane. Red phosphorus is one of the most popular phosphorus allotropes and is known to be a molecule derivative. This occurs in a non-crystalline network of phosphorus atoms. It is also considered to be more stable than white phosphor. Red phosphorus will act as a strong reducing agent.
The HI and red phosphorus acts as a reducing agent (reducing agent is usually located in one of the lowest possible oxidation states and is known as an electron donor and types of reducing agents include earth metals, formic acid, oxalic acid and sulfite compounds) and remove the iodine atom present and add hydrogen atom which forms the following compound.
During the reaction the oxygen present in the molecule gets removed as water molecule and instead of that a hydrogen atom takes its place which leads to formation of alkane/hydrocarbon.
Note:
Remember that the red phosphorus is highly harmful for humans, causing respiratory tract irritation, cough and various other problems. Hence, it is advised to use it carefully.
Complete Step By Step Answer:
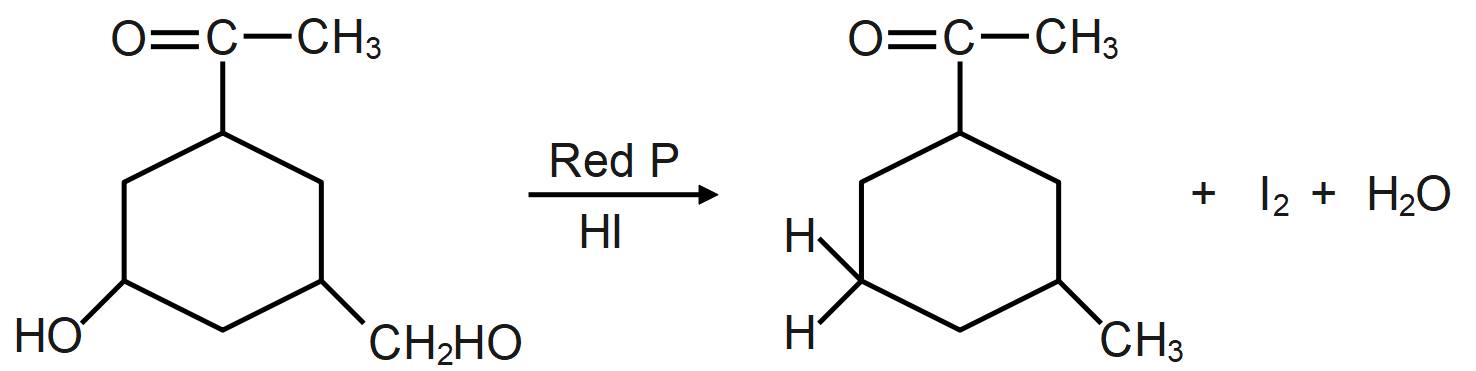
As we know, the reaction of a given compound with red phosphorus and HI results in the formation of alkane. Red phosphorus is one of the most popular phosphorus allotropes and is known to be a molecule derivative. This occurs in a non-crystalline network of phosphorus atoms. It is also considered to be more stable than white phosphor. Red phosphorus will act as a strong reducing agent.
The HI and red phosphorus acts as a reducing agent (reducing agent is usually located in one of the lowest possible oxidation states and is known as an electron donor and types of reducing agents include earth metals, formic acid, oxalic acid and sulfite compounds) and remove the iodine atom present and add hydrogen atom which forms the following compound.

During the reaction the oxygen present in the molecule gets removed as water molecule and instead of that a hydrogen atom takes its place which leads to formation of alkane/hydrocarbon.
Note:
Remember that the red phosphorus is highly harmful for humans, causing respiratory tract irritation, cough and various other problems. Hence, it is advised to use it carefully.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




