
Identify the products in the following reactions.
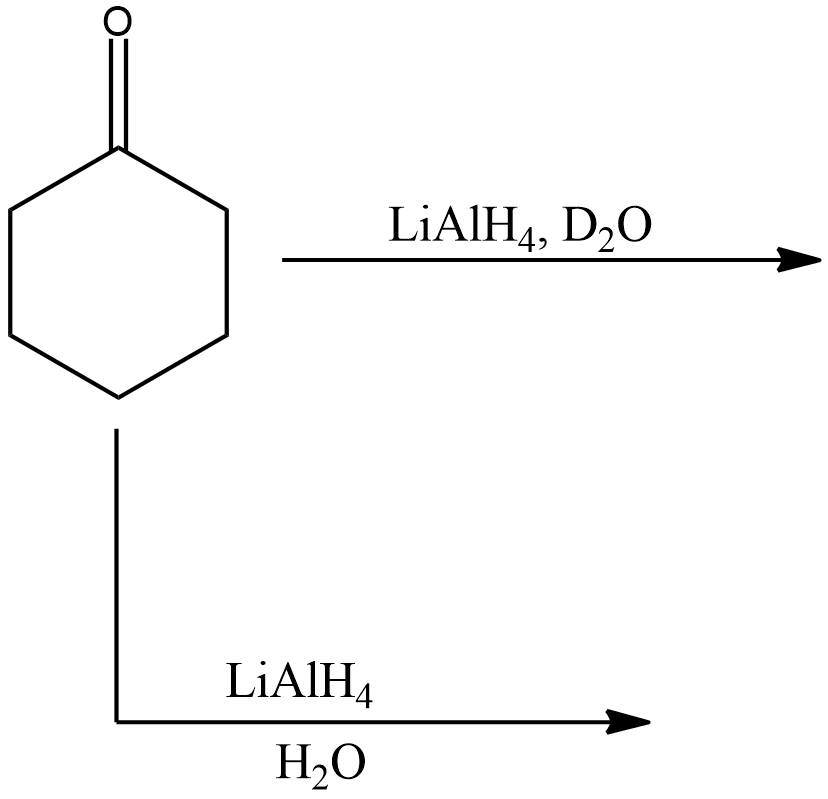

Answer
498k+ views
Hint: The reaction shown in the question is a reduction reaction. Lithium aluminium hydride is a reducing agent, thus the given compound will be reduced to alcohol. The compound given in the reaction is oxocyclohexane, commonly known as cyclohexanone.
Complete Step By Step Answer:
Let us first divide the two reactions into two different parts. The first reaction of cyclohexanone or oxocyclohexane is shown below.
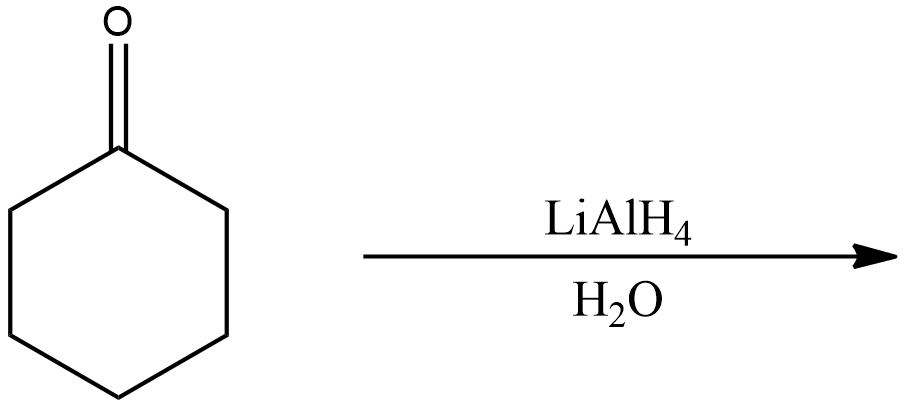
The compound given in the reaction is oxocyclohexane, it is subjected to reaction with Lithium aluminium hydride in presence of water. As we know that Lithium aluminium hydride is a reducing agent, the reaction will be a reduction reaction and the given compound (oxocyclohexane) will be reduced.
Now, the given compound (oxocyclohexane) consists of a carbonyl group. Thus, on reduction with Lithium aluminium hydride the carbonyl group will be reduced to hydroxyl group. Thus, forming an alcohol group on the compound, which is OH.
The reaction is shown below.
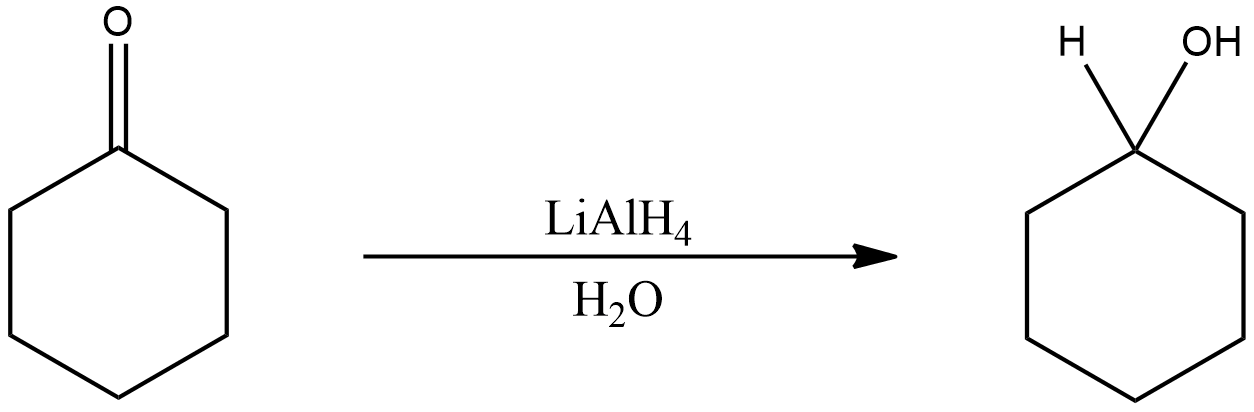
In this reaction, the hydroxyl group gets attached to the primary carbon along with the hydrogen. Thus forming cyclohexanol, cyclohexane with an alcohol group attached to it.
Now, for the second reaction,
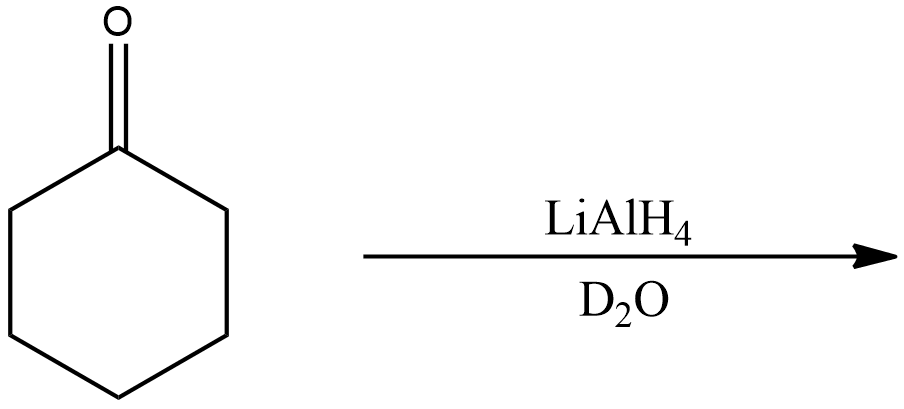
In this reaction, oxocyclohexane is subjected to reaction with Lithium aluminium hydride in presence of heavy water.
The reaction will be the same as above, just instead of water as a secondary reagent here heavy water is taken. Heavy water is nothing but the replacement of hydrogen with deuterium in water molecules. Deuterium is an isotope of hydrogen which is heavier than that, it consists of one proton, one electron and one neutron. Its atomic mass is 2 and its atomic number is 1.
Thus, the reaction of oxocyclohexane with Lithium aluminium hydride in presence of heavy water is shown below.
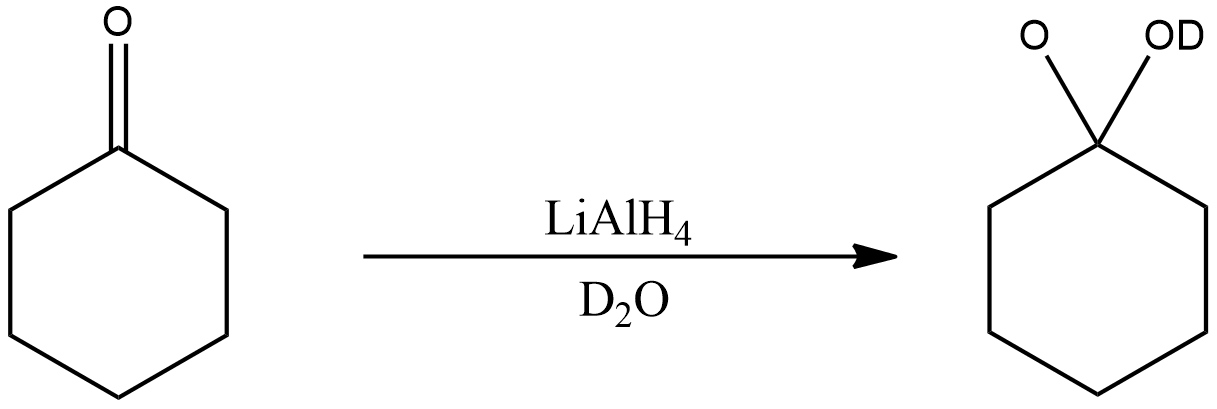
As shown in the figure, the compound (oxocyclohexane) is reduced to another compound in which deuterium oxide is attached to cyclohexane. As it can be seen, this reaction is similar to the previous reaction.
Note:
Deuterium dioxide or heavy water is drinkable, but only when it is mixed with normal water below fifty percent. More than fifty percent of heavy water dissolved in normal water can be really harmful. Lithium aluminium hydride and sodium borohydride are both strong reducing agents.
Complete Step By Step Answer:
Let us first divide the two reactions into two different parts. The first reaction of cyclohexanone or oxocyclohexane is shown below.
The compound given in the reaction is oxocyclohexane, it is subjected to reaction with Lithium aluminium hydride in presence of water. As we know that Lithium aluminium hydride is a reducing agent, the reaction will be a reduction reaction and the given compound (oxocyclohexane) will be reduced.
Now, the given compound (oxocyclohexane) consists of a carbonyl group. Thus, on reduction with Lithium aluminium hydride the carbonyl group will be reduced to hydroxyl group. Thus, forming an alcohol group on the compound, which is OH.
The reaction is shown below.
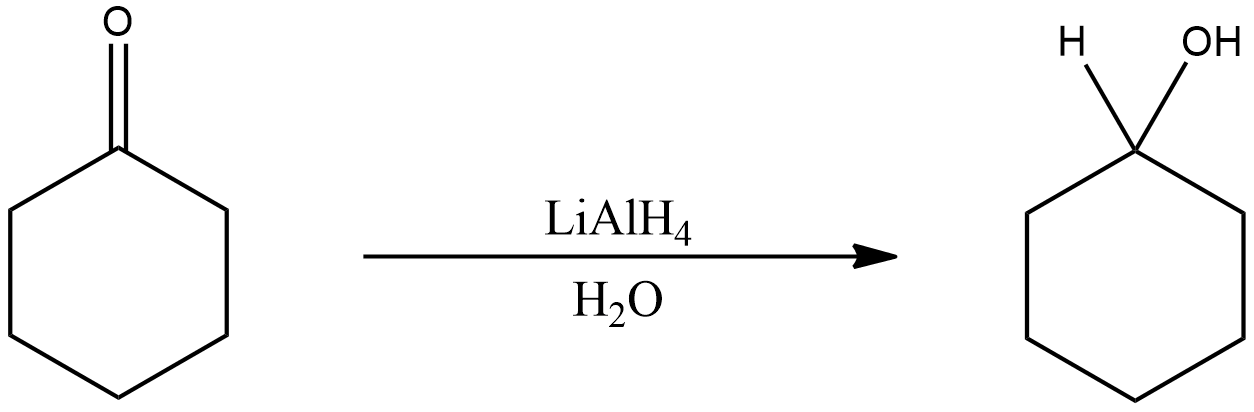
In this reaction, the hydroxyl group gets attached to the primary carbon along with the hydrogen. Thus forming cyclohexanol, cyclohexane with an alcohol group attached to it.
Now, for the second reaction,
In this reaction, oxocyclohexane is subjected to reaction with Lithium aluminium hydride in presence of heavy water.
The reaction will be the same as above, just instead of water as a secondary reagent here heavy water is taken. Heavy water is nothing but the replacement of hydrogen with deuterium in water molecules. Deuterium is an isotope of hydrogen which is heavier than that, it consists of one proton, one electron and one neutron. Its atomic mass is 2 and its atomic number is 1.
Thus, the reaction of oxocyclohexane with Lithium aluminium hydride in presence of heavy water is shown below.
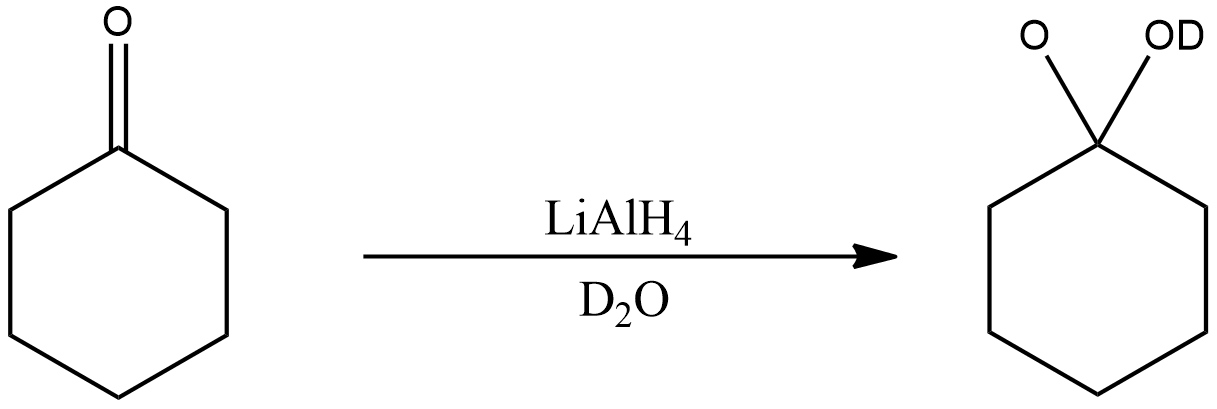
As shown in the figure, the compound (oxocyclohexane) is reduced to another compound in which deuterium oxide is attached to cyclohexane. As it can be seen, this reaction is similar to the previous reaction.
Note:
Deuterium dioxide or heavy water is drinkable, but only when it is mixed with normal water below fifty percent. More than fifty percent of heavy water dissolved in normal water can be really harmful. Lithium aluminium hydride and sodium borohydride are both strong reducing agents.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




