
If 2-chloro 3-methylpentane is treated with ethanolic KOH solution. How many different alkenes would be formed via \[{E_2}\] elimination reaction?
Answer
569.4k+ views
Hint: In this reaction, we will see the \[\beta - \]hydrohalogenation. This means that a HX group will undergo this reaction and leave and give us different alkenes as products.
Complete step by step answer:
-In the \[{E_2}\] mechanism, a base abstracts a proton neighboring the leaving group, forcing the electrons down to make a double bond, and, in so doing, forcing off the leaving group.
-When numerous things happen simultaneously in a mechanism, such as the \[{E_2}\] reaction, it is called a concerted step.
-Now let us see the particular compound given to us:
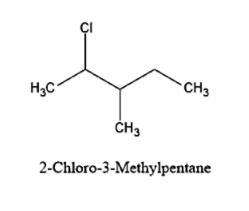
So, now with the reaction with alcoholic KOH, if favours \[\beta - \]dehydrohalogenation.
-So now let us understand what is \[\beta - \]dehydrohalogenation. The \[\beta - \]positions to the halide group in the compounds are those carbons just next to them. The carbon containing Cl is \[\alpha \] carbon while the other two carbon on its left and right are \[\beta - \]carbons.
-Now, the elimination of -HX group should be from any of these \[\beta - \] carbons.
But how to decide?
-Thus, we follow Saytzeff's rule here.
-It states that more substituted alkene will always favour hydrogen elimination from that particular \[\beta - \] carbon which has a lesser number of hydrogens.
-Then, the products formed are:
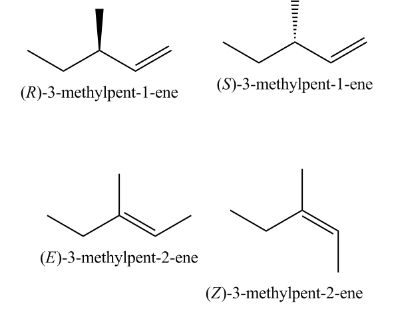
-According to Satyzeff rule, we can see that in the major product the elimination occurred from the \[\beta - \]carbon containing a lesser number of hydrogens.
-Since, this is an \[{E_2}\] reaction, so no question of formation of carbocation. The intermediates form products.
Therefore, the correct answer is 4.
Note: Always follow Saytzeff rule when in \[{E_2}\] elimination. In \[{E_2}\], the steps are all concerted while for \[{E_1}\], the steps involving carbocation formations are more stepwise. It depends more on the stability of the carbocation but that is not the scene for \[{E_2}\] eliminations.
Complete step by step answer:
-In the \[{E_2}\] mechanism, a base abstracts a proton neighboring the leaving group, forcing the electrons down to make a double bond, and, in so doing, forcing off the leaving group.
-When numerous things happen simultaneously in a mechanism, such as the \[{E_2}\] reaction, it is called a concerted step.
-Now let us see the particular compound given to us:

So, now with the reaction with alcoholic KOH, if favours \[\beta - \]dehydrohalogenation.
-So now let us understand what is \[\beta - \]dehydrohalogenation. The \[\beta - \]positions to the halide group in the compounds are those carbons just next to them. The carbon containing Cl is \[\alpha \] carbon while the other two carbon on its left and right are \[\beta - \]carbons.
-Now, the elimination of -HX group should be from any of these \[\beta - \] carbons.
But how to decide?
-Thus, we follow Saytzeff's rule here.
-It states that more substituted alkene will always favour hydrogen elimination from that particular \[\beta - \] carbon which has a lesser number of hydrogens.
-Then, the products formed are:

-According to Satyzeff rule, we can see that in the major product the elimination occurred from the \[\beta - \]carbon containing a lesser number of hydrogens.
-Since, this is an \[{E_2}\] reaction, so no question of formation of carbocation. The intermediates form products.
Therefore, the correct answer is 4.
Note: Always follow Saytzeff rule when in \[{E_2}\] elimination. In \[{E_2}\], the steps are all concerted while for \[{E_1}\], the steps involving carbocation formations are more stepwise. It depends more on the stability of the carbocation but that is not the scene for \[{E_2}\] eliminations.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




