
If reaction \[A \to B\] is exothermic, how does the activation energy for the forward reaction compare with the activation energy of the reverse reaction, \[B \to A\]?
Answer
558.9k+ views
Hint: In order to compare the activation energy of the forward and the reverse reaction, we must first have an idea about what an activation energy is. Activation energy is referred to as the minimum amount of the extra energy which is required by a reacting molecule to get converted into a product.
Complete step by step answer:
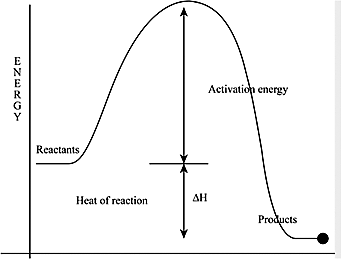
- Let us understand about the activation energy. Activation energy is referred to as the minimum amount of the extra energy which is required by a reacting molecule to get converted into a product. In other words, we can say that it is the minimum amount of energy which is required to energize or activate the atoms or molecules for a chemical reaction to take place.
- The given forward reaction \[A \to B\] is exothermic. The activation energy for the forward reaction is found to be smaller than the reverse reaction.
Therefore, the forward reaction can be written as the :
\[A \to B + heat\]
The product formed will be lower than the reactant as the heat is released during the reaction. We can move from a higher energy level in case of A to a lower energy level in case of B. The difference in the energy between A and B is the enthalpy change of the reaction.
In the reverse reaction:
\[heat + B \to A\]
We have to supply energy to the more stable B to make it to reform the less stable A which is in the higher energy level.
The activation energy difference between the forward and the reverse reaction will be \[\Delta H\].
Therefore, we can write as :
\[{E_{a{\text{ reverse}}}} = \Delta H + {E_{a{\text{ forward}}}}\]
We can finally say that the activation energy of the reverse reaction is greater than the activation energy of the forward reaction.
Note: We have to remember that the exothermic reaction is different from the endothermic reaction. The reaction which can release the energy from the system in the form of heat is called the exothermic reaction. The reaction in which the system can absorb the energy in the form of heat from the surrounding is called the endothermic reaction.
Complete step by step answer:
- Let us understand about the activation energy. Activation energy is referred to as the minimum amount of the extra energy which is required by a reacting molecule to get converted into a product. In other words, we can say that it is the minimum amount of energy which is required to energize or activate the atoms or molecules for a chemical reaction to take place.
- The given forward reaction \[A \to B\] is exothermic. The activation energy for the forward reaction is found to be smaller than the reverse reaction.
Therefore, the forward reaction can be written as the :
\[A \to B + heat\]
The product formed will be lower than the reactant as the heat is released during the reaction. We can move from a higher energy level in case of A to a lower energy level in case of B. The difference in the energy between A and B is the enthalpy change of the reaction.
In the reverse reaction:
\[heat + B \to A\]
We have to supply energy to the more stable B to make it to reform the less stable A which is in the higher energy level.
The activation energy difference between the forward and the reverse reaction will be \[\Delta H\].
Therefore, we can write as :
\[{E_{a{\text{ reverse}}}} = \Delta H + {E_{a{\text{ forward}}}}\]
We can finally say that the activation energy of the reverse reaction is greater than the activation energy of the forward reaction.

Note: We have to remember that the exothermic reaction is different from the endothermic reaction. The reaction which can release the energy from the system in the form of heat is called the exothermic reaction. The reaction in which the system can absorb the energy in the form of heat from the surrounding is called the endothermic reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




