
In a cubic structure of diamond which is made from $X$ and $Y$ , where $X$ atoms are at the corners of the cube and $Y$ at the face centres of the cube . The molecular formula of the compound is :
A: ${X_2}Y$
B: ${X_3}Y$
C: $X{Y_2}$
D: $X{Y_3}$
Answer
583.2k+ views
Hint:Whenever we are given a solid cube of a molecule , the positions of the substituent atoms determine the molecular formula of the compound. If the atom is in the edge centre it contributes \[\]$\dfrac{1}{4}$ to the particular molecule , if it is in the face centre it contributes $\dfrac{1}{2}$ to the molecule and if it is in the corner of the cube it contributes $\dfrac{1}{8}$ to the molecule.
Complete step by step answer:
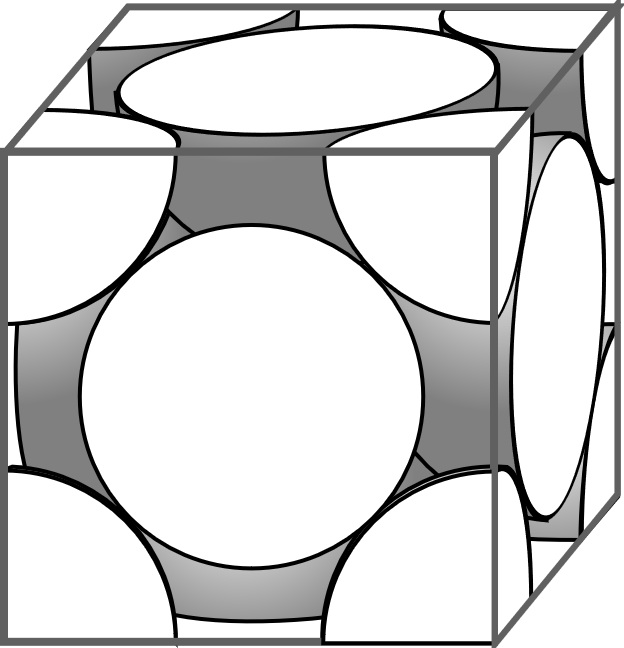
In the given question it is given that the $X$ atoms are in the corners and the $Y$ atoms are in the face centre. In a given cube there are eight corners and six faces. So there will be eight $X$ atoms in the eight corners of the cube and six $Y$ atoms in the six faces of the cube. The contribution of the corner atoms to a cube is $\dfrac{1}{8}$ to the compound and the contribution of the face centred atoms to the compound is $\dfrac{1}{2}$ . So the molecular formula of the compound will be
${X_{\dfrac{1}{8}}}_{ \times 8}{Y_{\dfrac{1}{2}}}_{ \times 6} = X{Y_3}$
So from a the above explanation and calculation it is clear that the correct answer of the question is
D: $X{Y_3}$
Additional information : When a cube has eight atoms in eight corners and one atom in the body centre it is known as body centred cube. When a cube has eight atoms in eight corners and six atoms in the six faces of the cube it is known as a face centred cube.
Note: Always remember that the atoms in the corner constitute $\dfrac{1}{8}$ the compound , atoms in faces contributes $\dfrac{1}{2}$ ,atoms in the edge centre contributes $\dfrac{1}{4}$ and atoms in body centre contributes 1 to the compound. To find the molecular formula of the compound multiply the number of atoms in the particular positions with their respective contribution to the compound.
Complete step by step answer:

In the given question it is given that the $X$ atoms are in the corners and the $Y$ atoms are in the face centre. In a given cube there are eight corners and six faces. So there will be eight $X$ atoms in the eight corners of the cube and six $Y$ atoms in the six faces of the cube. The contribution of the corner atoms to a cube is $\dfrac{1}{8}$ to the compound and the contribution of the face centred atoms to the compound is $\dfrac{1}{2}$ . So the molecular formula of the compound will be
${X_{\dfrac{1}{8}}}_{ \times 8}{Y_{\dfrac{1}{2}}}_{ \times 6} = X{Y_3}$
So from a the above explanation and calculation it is clear that the correct answer of the question is
D: $X{Y_3}$
Additional information : When a cube has eight atoms in eight corners and one atom in the body centre it is known as body centred cube. When a cube has eight atoms in eight corners and six atoms in the six faces of the cube it is known as a face centred cube.
Note: Always remember that the atoms in the corner constitute $\dfrac{1}{8}$ the compound , atoms in faces contributes $\dfrac{1}{2}$ ,atoms in the edge centre contributes $\dfrac{1}{4}$ and atoms in body centre contributes 1 to the compound. To find the molecular formula of the compound multiply the number of atoms in the particular positions with their respective contribution to the compound.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




