
In a heat engine, heat energy is converted to mechanical energy.
A). True
B). False
Answer
600.6k+ views
Hint: According to the first law of Thermodynamics, Energy can neither be created nor destroyed, only altered from one form into the other. Thus conversion of energy from one form to another is possible. Furthermore, Heat engine is a device that is used to convert heat energy into mechanical work by using the temperature difference. Hence the understanding of the definition of the device is sufficient to answer this question.
Complete step by step answer:
When mechanical work is done on a system, its internal energy increases.
The reverse process, that is when mechanical work is obtained at the expense of internal energy, is performed by the means of a device known as Heat engine.
Heat engines perform this task of conversion of energy from one form into the other at the expense of using temperature difference between two bodies. The body at higher temperature is called a “source” and the body at lower temperature is called a “sink”.
The basic task of a heat engine is to take some heat energy from bodies at higher temperature and convert a part of it into mechanical work and deliver the rest of this energy in the form of heat itself to the body at lower temperature.
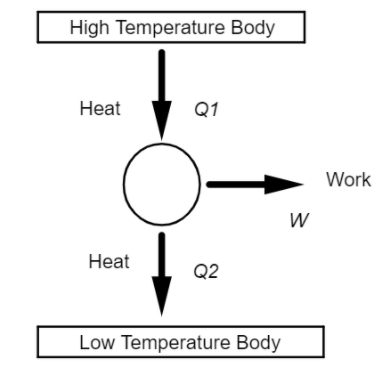
The above diagram shows the basic activity of a heat engine,
Where ${Q_1}$ is the amount of heat taken by the heat engine from the higher temperature body,
W is the part of heat energy converted by the heat engine into Work, that is mechanical energy,
${Q_2}$ is the heat rejected by the heat engine to the lower temperature body.
Thus, the given statement is true that in a heat engine, heat energy is converted to mechanical energy.
Hence the correct answer is A, i.e. True.
Additional Information: Heat engines are a very important device. The efficiency of a heat engine describes how good the heat engine is in performing its required task of energy conversion.
Let ${Q_1}$
${Q_2}$, W represent the same physical quantities as mentioned above.
Additionally, if the final state of the substance inside the engine is the same as the initial state, there is no change in its internal energy.
By first law of thermodynamics, i.e, Energy can neither be created nor destroyed, only altered from one form into the other.
Mathematically,
$\eqalign{
& \Delta Q = \Delta U + \Delta W \cr
& \Rightarrow W = {Q_1} - {Q_2} \cr} $
Therefore, efficiency of a heat engine is given by:
$\eqalign{
& \eta = \dfrac{{{\text{Work done by the engine}}}}{{{\text{heat supplied to it}}}} \cr
& \eta = \dfrac{W}{{{Q_1}}} = \dfrac{{{Q_1} - {Q_2}}}{{{Q_1}}} = 1 - \dfrac{{{Q_2}}}{{{Q_1}}} \cr} $
Note: By the first law of thermodynamics it is sure that energy can be converted from one form into the other. Thus it is possible to convert the heat energy into mechanical energy. Afterwards just the proper understanding of what a heat engine is necessary. Many times students usually try to memorize the definitions just for the sake of it. Rather they should focus on understanding the definition and the concept behind it. Then the answer would be clear to them within seconds.
Complete step by step answer:
When mechanical work is done on a system, its internal energy increases.
The reverse process, that is when mechanical work is obtained at the expense of internal energy, is performed by the means of a device known as Heat engine.
Heat engines perform this task of conversion of energy from one form into the other at the expense of using temperature difference between two bodies. The body at higher temperature is called a “source” and the body at lower temperature is called a “sink”.
The basic task of a heat engine is to take some heat energy from bodies at higher temperature and convert a part of it into mechanical work and deliver the rest of this energy in the form of heat itself to the body at lower temperature.

The above diagram shows the basic activity of a heat engine,
Where ${Q_1}$ is the amount of heat taken by the heat engine from the higher temperature body,
W is the part of heat energy converted by the heat engine into Work, that is mechanical energy,
${Q_2}$ is the heat rejected by the heat engine to the lower temperature body.
Thus, the given statement is true that in a heat engine, heat energy is converted to mechanical energy.
Hence the correct answer is A, i.e. True.
Additional Information: Heat engines are a very important device. The efficiency of a heat engine describes how good the heat engine is in performing its required task of energy conversion.
Let ${Q_1}$
${Q_2}$, W represent the same physical quantities as mentioned above.
Additionally, if the final state of the substance inside the engine is the same as the initial state, there is no change in its internal energy.
By first law of thermodynamics, i.e, Energy can neither be created nor destroyed, only altered from one form into the other.
Mathematically,
$\eqalign{
& \Delta Q = \Delta U + \Delta W \cr
& \Rightarrow W = {Q_1} - {Q_2} \cr} $
Therefore, efficiency of a heat engine is given by:
$\eqalign{
& \eta = \dfrac{{{\text{Work done by the engine}}}}{{{\text{heat supplied to it}}}} \cr
& \eta = \dfrac{W}{{{Q_1}}} = \dfrac{{{Q_1} - {Q_2}}}{{{Q_1}}} = 1 - \dfrac{{{Q_2}}}{{{Q_1}}} \cr} $
Note: By the first law of thermodynamics it is sure that energy can be converted from one form into the other. Thus it is possible to convert the heat energy into mechanical energy. Afterwards just the proper understanding of what a heat engine is necessary. Many times students usually try to memorize the definitions just for the sake of it. Rather they should focus on understanding the definition and the concept behind it. Then the answer would be clear to them within seconds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




