
In a simple cubic lattice, the coordination number is:
A. \[4\]
B. \[5\]
C. \[6\]
D. \[8\]
Answer
585.9k+ views
Hint: In order to answer this question we must have an idea about crystal lattice mainly simple cubic lattice.
Here coordination number of lattice means total number of surrounding particles present around one particle.
Complete step by step answer:
Among three states of matter (solid, liquid, and gas ) solid has crystal lattice structure which is three-dimensional regular arrangement of units cell of lattice points (constituent particles may be atoms, ions, or molecules)
Now let us imagine that a cube that has eight particles at eight corners as closely packed, in particles, are attached to one another. This type is classified as a unit cell of simple cubic lattice.
Figure is shown below.
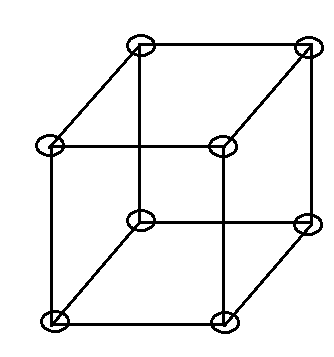
For our convenience to present a clear picture we don’t show here the closed packed structure.
Now we have to look forward to coordination number total no. of nearest surrounding particles present. For a simple cubic lattice, it is 6. Which we can understand from the figure below.
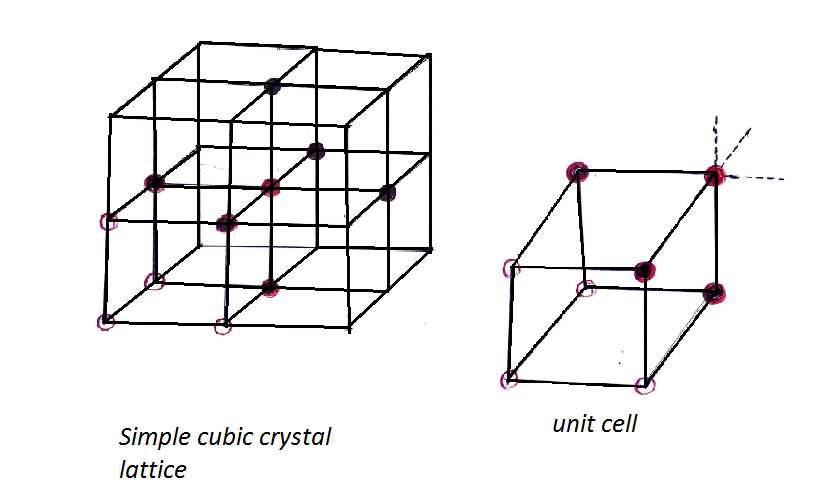
The coordination number of a simple cubic crystal \[\left( {SCC} \right)\] lattice is 6.
So, the correct answer is “Option C”.
Note:
To understand clearly we have to concentrate on any corner particle which is shared with adjacent eight-unit cells.
The unit cell of a simple cubic crystal \[\left( {SCC} \right)\]lattice has \[8\left( {eight} \right)\] corner particles, six faces twelve edges.
The coordination number of others lattice as example body-centered cubic lattice is \[8\left( {eight} \right)\]and face-centered cubic .\[\left( {FCC} \right)\].
Here coordination number of lattice means total number of surrounding particles present around one particle.
Complete step by step answer:
Among three states of matter (solid, liquid, and gas ) solid has crystal lattice structure which is three-dimensional regular arrangement of units cell of lattice points (constituent particles may be atoms, ions, or molecules)
Now let us imagine that a cube that has eight particles at eight corners as closely packed, in particles, are attached to one another. This type is classified as a unit cell of simple cubic lattice.
Figure is shown below.

For our convenience to present a clear picture we don’t show here the closed packed structure.
Now we have to look forward to coordination number total no. of nearest surrounding particles present. For a simple cubic lattice, it is 6. Which we can understand from the figure below.

The coordination number of a simple cubic crystal \[\left( {SCC} \right)\] lattice is 6.
So, the correct answer is “Option C”.
Note:
To understand clearly we have to concentrate on any corner particle which is shared with adjacent eight-unit cells.
The unit cell of a simple cubic crystal \[\left( {SCC} \right)\]lattice has \[8\left( {eight} \right)\] corner particles, six faces twelve edges.
The coordination number of others lattice as example body-centered cubic lattice is \[8\left( {eight} \right)\]and face-centered cubic .\[\left( {FCC} \right)\].
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




