
In $ A{l_2}C{l_6} $ , the number of covalent and coordinate bonds are _______ respectively.
A. 3,3
B. 2,4
C. 6,3
D. 6,0
Answer
544.5k+ views
Hint: Initially we must have a prior knowledge of bonding and types of bond. Then you have to draw the structure and you can easily calculate the number of covalent and coordinate bonds. Covalent bond is the number of valence electrons.
Complete step by step solution:
Chemical Bonding refers to the formation of a chemical bond between two or more atoms, molecules, or ions to give rise to a chemical compound.
A covalent bond is formed between two atoms by sharing electrons. The number of bonds an element forms in a covalent compound is determined by the number of electrons it needs to reach octet.
There is also a formula that fails in many cases but may sometimes become handy: Formal Charge = (Oxidation State of the central atom - 8 + Digit of its group number)/2
Structure of $ A{l_2}C{l_6} $
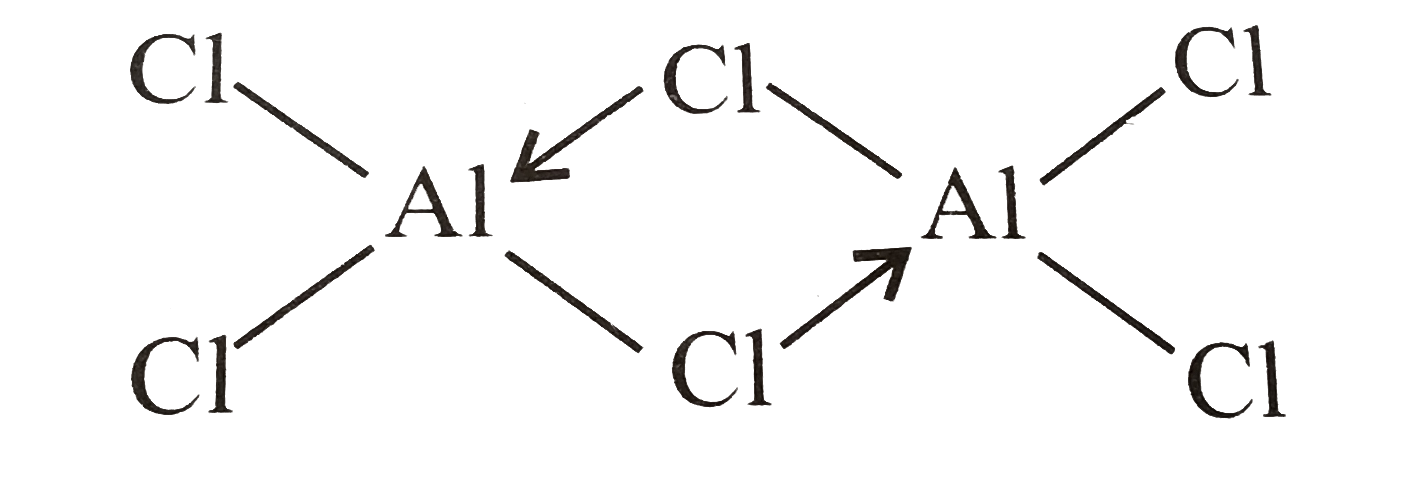
It is clearly visible that the number of covalent and coordinate bonds are 6 and 3.
Note:
Three types of chemical bond are:
Ionic bond, coordinate bond, covalent bond.
Ionic bond: - The ionic bond is the electrostatic force of attraction between two oppositely charged ions i.e., a positively charged cation and a negatively charged anion.
Covalent bond: - A covalent bond is formed by equal sharing of electrons from both the participating atoms. The pair of electrons participating in this type of bonding is called a shared pair or bonding pair.
Coordinate bond: - Co-ordinate bond is a type of alternate covalent bond that is formed by sharing an electron pair from a single atom.
Complete step by step solution:
Chemical Bonding refers to the formation of a chemical bond between two or more atoms, molecules, or ions to give rise to a chemical compound.
A covalent bond is formed between two atoms by sharing electrons. The number of bonds an element forms in a covalent compound is determined by the number of electrons it needs to reach octet.
There is also a formula that fails in many cases but may sometimes become handy: Formal Charge = (Oxidation State of the central atom - 8 + Digit of its group number)/2
Structure of $ A{l_2}C{l_6} $

It is clearly visible that the number of covalent and coordinate bonds are 6 and 3.
Note:
Three types of chemical bond are:
Ionic bond, coordinate bond, covalent bond.
Ionic bond: - The ionic bond is the electrostatic force of attraction between two oppositely charged ions i.e., a positively charged cation and a negatively charged anion.
Covalent bond: - A covalent bond is formed by equal sharing of electrons from both the participating atoms. The pair of electrons participating in this type of bonding is called a shared pair or bonding pair.
Coordinate bond: - Co-ordinate bond is a type of alternate covalent bond that is formed by sharing an electron pair from a single atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




