
In allene structure, three carbon atoms are joined by:
\[\] \[\begin{array}{*{35}{l}}
\left( \text{A} \right)\text{ three }\sigma \text{and three }\pi \text{ bond} \\
\left( \text{B} \right)\text{ two }\sigma \text{ and one }\pi \text{ bond} \\
\left( \text{C } \right)\text{ two }\sigma \text{ and two }\pi \text{ bonds} \\
\left( \text{D} \right)\text{ three }\pi \text{ bonds only} \\
\end{array}\]
Answer
592.2k+ views
Hint: Ene indicates an alkene group which confirms presence of one double bond and allene are organic compounds in which one central carbon atom has double bonds with adjacent carbon atoms.
Complete step by step answer:
- Allene refers to structural formula C=C=C
- Allene indicates presence of double bond, so structural formula of ALLENE is H2C=C=CH2
- The Hybridization of the Central Carbon atom in allene is sp. Hybridization of terminal carbon atoms is $s{{p}^{2}}$.
- The Electronic configuration of carbon atom in ground state is $1{{s}^{2}}2{{s}^{2}}2{{p}^{2}}$.
- Valency of carbon atoms is 4, so an electron from 2s is transferred to 2p as the energy level of 2s and 2p is -almost the same.
- The electronic configuration of carbon atoms in excited state is $1{{s}^{2}}2{{s}^{1}}2{{p}^{3}}$.
- As Energy level of 2s and 2p is almost same, so 2s orbital and one p orbital mix together to form two sp hybridized orbitals which overlap with orbitals of other atom to form sigma bonds and remaining 2p orbitals are unhybridized which overlap sidewise to form two pi bonds.
- For terminal carbon atoms, one 2s orbital and two 2p form three $s{{p}^{2}}$ orbitals and one p orbital remains unhybridized.
- A double bond has one sigma and one pi bond.
- As there are two double bonds between three carbon atoms in allene, there are two sigma bonds and two pi bonds between them.
So option C is correct.
Note: Single bond is sigma bond. Double bond has one sigma plus one pi bond. Triple bond has one sigma and two pi bonds. Allene is confused with Allyl which is having the structural formula H2C=CH-CH2R.
Allene is also known as Propadiene as prop indicates it has three carbons and Diene indicates two double bonds.
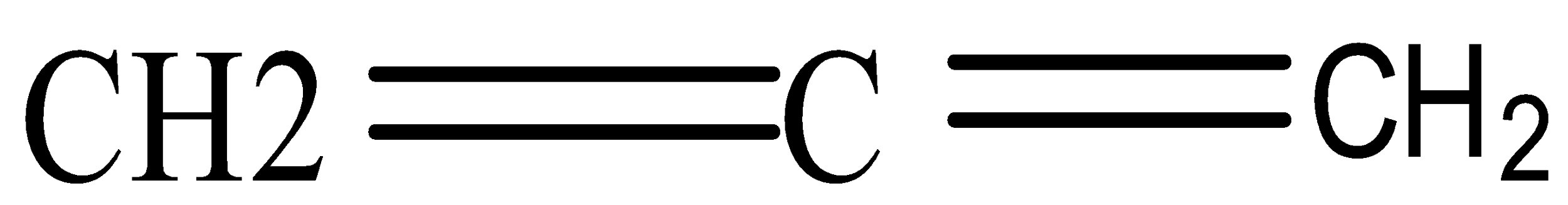
Complete step by step answer:
- Allene refers to structural formula C=C=C
- Allene indicates presence of double bond, so structural formula of ALLENE is H2C=C=CH2
- The Hybridization of the Central Carbon atom in allene is sp. Hybridization of terminal carbon atoms is $s{{p}^{2}}$.
- The Electronic configuration of carbon atom in ground state is $1{{s}^{2}}2{{s}^{2}}2{{p}^{2}}$.
- Valency of carbon atoms is 4, so an electron from 2s is transferred to 2p as the energy level of 2s and 2p is -almost the same.
- The electronic configuration of carbon atoms in excited state is $1{{s}^{2}}2{{s}^{1}}2{{p}^{3}}$.
- As Energy level of 2s and 2p is almost same, so 2s orbital and one p orbital mix together to form two sp hybridized orbitals which overlap with orbitals of other atom to form sigma bonds and remaining 2p orbitals are unhybridized which overlap sidewise to form two pi bonds.
- For terminal carbon atoms, one 2s orbital and two 2p form three $s{{p}^{2}}$ orbitals and one p orbital remains unhybridized.
- A double bond has one sigma and one pi bond.
- As there are two double bonds between three carbon atoms in allene, there are two sigma bonds and two pi bonds between them.
So option C is correct.
Note: Single bond is sigma bond. Double bond has one sigma plus one pi bond. Triple bond has one sigma and two pi bonds. Allene is confused with Allyl which is having the structural formula H2C=CH-CH2R.
Allene is also known as Propadiene as prop indicates it has three carbons and Diene indicates two double bonds.
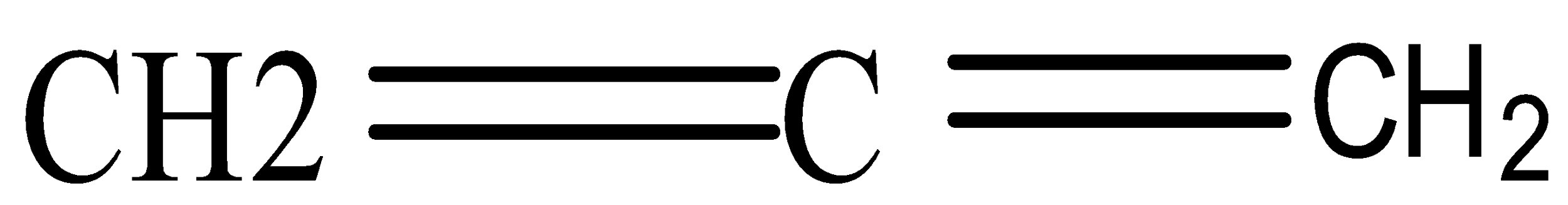
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




