
In an electrolytic cell current flows from?
A.Cathode to anode in outer circuit
B.Anode to cathode outside the cell
C.Cathode to anode inside the cell
D.Anode to cathode inside the cell.
Answer
497.1k+ views
Hint: An electrolytic cell is an electrochemical cell that uses electrical energy to drive a non-spontaneous redox reaction. It is often used to decompose chemical compounds, in a process called electrolysis and Electrons flow from anode to cathode.
Complete answer:
An electrolytic cell converts electrical energy into chemical energy. Here, the redox reaction is spontaneous and is responsible for the production of electrical energy. The reaction at the anode is oxidation and that at the cathode is reduction. The electrons are supplied by the species getting oxidized.
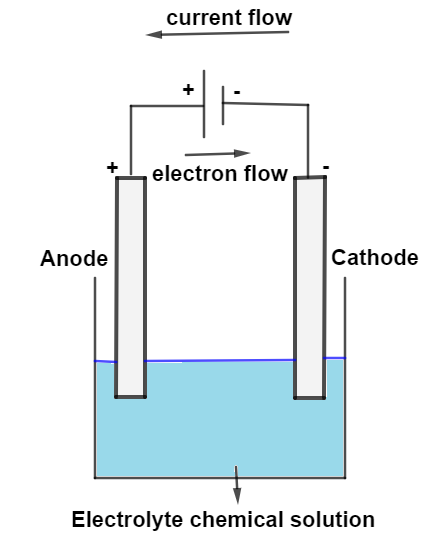
In an electrolytic cell, the negative terminal is the cathode/anode and is the site of the oxidation/reduction half reaction. In an electrolytic cell, the negative terminal is the cathode and is the site of the reduction half reaction. For an electrolytic cell however, this flow is not spontaneous but must be driven by an external power source. In an electrolytic cell, the anode has the "" sign. The potential difference between the electrodes causes electrons to flow from the reductant to the oxidant through the external circuit, generating an electric current.
So, the correct answer is (C) cathode to anode inside the cell.
Note:
Remember Current does not flow out of a battery. Current, a movement of electrons, flows from one part of the battery richer to the other part poorer of them. Batteries put out direct current, as opposed to alternating current, which is what comes out of a wall socket. With direct current, the charge flows only in one direction. With alternating current, the charges slosh back and forth, continually reversing direction.
Complete answer:
An electrolytic cell converts electrical energy into chemical energy. Here, the redox reaction is spontaneous and is responsible for the production of electrical energy. The reaction at the anode is oxidation and that at the cathode is reduction. The electrons are supplied by the species getting oxidized.

In an electrolytic cell, the negative terminal is the cathode/anode and is the site of the oxidation/reduction half reaction. In an electrolytic cell, the negative terminal is the cathode and is the site of the reduction half reaction. For an electrolytic cell however, this flow is not spontaneous but must be driven by an external power source. In an electrolytic cell, the anode has the "" sign. The potential difference between the electrodes causes electrons to flow from the reductant to the oxidant through the external circuit, generating an electric current.
So, the correct answer is (C) cathode to anode inside the cell.
Note:
Remember Current does not flow out of a battery. Current, a movement of electrons, flows from one part of the battery richer to the other part poorer of them. Batteries put out direct current, as opposed to alternating current, which is what comes out of a wall socket. With direct current, the charge flows only in one direction. With alternating current, the charges slosh back and forth, continually reversing direction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




