
In benzene, the triple bond consists of-
(A)- one sp-sp sigma bond and two p-p pi bonds
(B)- two sp-sp sigma bonds and one p-p pi bonds
(C)- one $s{{p}^{2}}-s{{p}^{2}}$ sigma bond and one p-p pi bond
(D)- one $s{{p}^{2}}-s{{p}^{2}}$ sigma bond, one$s{{p}^{2}}-s{{p}^{2}}$ pi bond and one p-p pi bond.
Answer
577.8k+ views
Hint: A triple bond in benzene is actually a benzene molecule. All three bonds are different in nature, two of them being coplanar and one out of the plane. The two bonds are the same as that of a normal benzene ring and the third one lies perpendicular to the p-orbitals of the aromatic pi system.
Complete step by step solution:
The structure of benzene and benzyne (benzene with a triple bond) are-
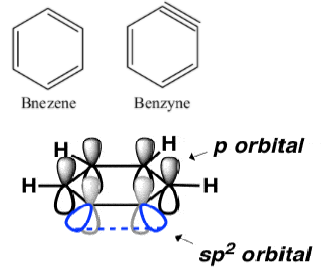
$s{{p}^{2}}$ Orbitals involved in the triple bond are at ${{90}^{\circ }}$ to the p- orbitals of the aromatic pi system involved. Additional pi bonds cannot overlap the aromatic pi system, hence they are not coplanar.
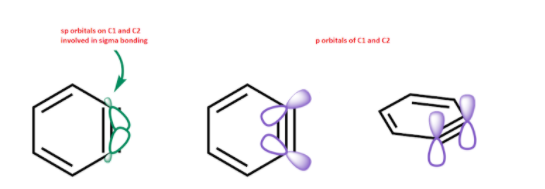
There is already a sigma $s{{p}^{2}}-s{{p}^{2}}$ bond between two carbon atoms. And a regular p-p pi bond as that present in a normal benzene ring.
So the correct answer is option (D)- one $s{{p}^{2}}-s{{p}^{2}}$ sigma bond, one$s{{p}^{2}}-s{{p}^{2}}$ pi bond and one p-p pi bond.
Additional information:
The triple bond in a six-membered ring makes benzyne a highly reactive molecule as poor overlap leads to a very weak triple bond, easily attacked by a nucleophile.
Note: One should note that the benzene bond is not like the triple bond of acetylene which is a very strong bond as the $s{{p}^{2}}$ orbitals are not parallel and therefore involve poor overlap in benzyne.
Complete step by step solution:
The structure of benzene and benzyne (benzene with a triple bond) are-

$s{{p}^{2}}$ Orbitals involved in the triple bond are at ${{90}^{\circ }}$ to the p- orbitals of the aromatic pi system involved. Additional pi bonds cannot overlap the aromatic pi system, hence they are not coplanar.

There is already a sigma $s{{p}^{2}}-s{{p}^{2}}$ bond between two carbon atoms. And a regular p-p pi bond as that present in a normal benzene ring.
So the correct answer is option (D)- one $s{{p}^{2}}-s{{p}^{2}}$ sigma bond, one$s{{p}^{2}}-s{{p}^{2}}$ pi bond and one p-p pi bond.
Additional information:
The triple bond in a six-membered ring makes benzyne a highly reactive molecule as poor overlap leads to a very weak triple bond, easily attacked by a nucleophile.
Note: One should note that the benzene bond is not like the triple bond of acetylene which is a very strong bond as the $s{{p}^{2}}$ orbitals are not parallel and therefore involve poor overlap in benzyne.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




