
In benzene, what is the hybridization on each carbon atom?
A)${\text{s}}{{\text{p}}^2}$
B)${\text{s}}{{\text{p}}^3}$
C)${\text{s}}{{\text{p}}^3}{\text{d}}$
D)${\text{sp}}$
Answer
591.9k+ views
Hint: In benzene each carbon atom is joined to three other atoms-one hydrogen and two carbons. The carbon atom in benzene lacks the number of unpaired electrons to form bonds.
Complete step by step answer:
-The formula of benzene is ${{\text{C}}_6}{{\text{H}}_6}$.It is a six carbon ring with three alternate double bonds.
-At ground state the electronic configuration of carbon is $1{s^2}2{s^2}2p_x^12p_y^1$ -Since it lacks number of unpaired electron so it will promote one of the $2{s^2}$ electrons to jump into $2p_z^{}$ orbital.
-In excited state the electronic configuration of carbon will become- $1{s^2}2{s^1}2p_x^12p_y^12p_z^1$.So we get $4$ unpaired electrons and these electrons will participate in bond formation.
- The carbon atoms hybridize their outer orbital before forming the bonds as they have only three orbitals to hybridize. So they use $2{\text{s}}$ and two $2p$ orbitals to hybridize to form $3$ ${\text{s}}{{\text{p}}^2}$ hybrid orbitals and leave the other $2p{\text{ }}$ electrons unchanged.
- The three ${\text{s}}{{\text{p}}^2}$ hybrid orbitals arrange themselves at ${120^ \circ }$ to each other in the plane. Out of the three hybrid orbitals two orbitals overlap axially with orbitals of the neighboring carbon atoms on the both sides to form sigma bonds.
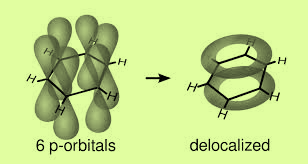
The left p orbital overlap sidewise with similar orbitals of the other carbon atoms on either side to form two sets of pi-bonds. So the resultant pi-cloud is formed over all the six carbon atoms. This is known as the delocalization of electron charge. Benzene has trigonal planar geometry.
So the correct answer is A.
Note:
Benzene has following applications-
1.It is used to make plastics, resins, synthetic fibers.
2. It is used to prepare detergents, dyes, drugs and pesticides.
Complete step by step answer:
-The formula of benzene is ${{\text{C}}_6}{{\text{H}}_6}$.It is a six carbon ring with three alternate double bonds.
-At ground state the electronic configuration of carbon is $1{s^2}2{s^2}2p_x^12p_y^1$ -Since it lacks number of unpaired electron so it will promote one of the $2{s^2}$ electrons to jump into $2p_z^{}$ orbital.
-In excited state the electronic configuration of carbon will become- $1{s^2}2{s^1}2p_x^12p_y^12p_z^1$.So we get $4$ unpaired electrons and these electrons will participate in bond formation.
- The carbon atoms hybridize their outer orbital before forming the bonds as they have only three orbitals to hybridize. So they use $2{\text{s}}$ and two $2p$ orbitals to hybridize to form $3$ ${\text{s}}{{\text{p}}^2}$ hybrid orbitals and leave the other $2p{\text{ }}$ electrons unchanged.
- The three ${\text{s}}{{\text{p}}^2}$ hybrid orbitals arrange themselves at ${120^ \circ }$ to each other in the plane. Out of the three hybrid orbitals two orbitals overlap axially with orbitals of the neighboring carbon atoms on the both sides to form sigma bonds.

The left p orbital overlap sidewise with similar orbitals of the other carbon atoms on either side to form two sets of pi-bonds. So the resultant pi-cloud is formed over all the six carbon atoms. This is known as the delocalization of electron charge. Benzene has trigonal planar geometry.
So the correct answer is A.
Note:
Benzene has following applications-
1.It is used to make plastics, resins, synthetic fibers.
2. It is used to prepare detergents, dyes, drugs and pesticides.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




