
In bisulphate ion, the formal charge on the sulfur atom is:
A. \[+1\]
B. \[+2\]
C. \[+4\]
D. \[+6\]
Answer
569.7k+ views
Hint: Formal charge of an atom in a molecule can be found by the relation: $formal\,charge=valence\text{ }electrons-\left( non-bonding\text{ }valance\text{ }electrons \right)-\dfrac{\left( bonding\text{ }electrons \right)}{2}$
Using the above relation, find the formal charge on the sulfur atom in bisulphate ion.
Complete step by step answer:
We know that the relation to find the formal charge mathematical can be expressed as shown below:
$formal\,charge=valence\text{ }electrons-\left( non-bonding\text{ }valance\text{ }electrons \right)-\dfrac{\left( bonding\text{ }electrons \right)}{2}$
The structure of the bisulphate ion is as follows:
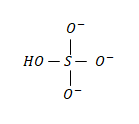
In which the middle atom is the sulfur atom.
The valence electrons of the sulfur atom is = 06 electrons;
The non-bonding valence electrons of the sulfur atom in the above molecule is = 0 electrons;
The bonding valence electrons of the sulfur atom in the above molecule is = 08 electrons.
Put all these values in the above formula and calculate the formal charge
After substituting all the values in the above relation we obtain
$formal\,charge=6-\left( 0 \right)-\dfrac{8}{2}\text{ = +2 }$
Therefore the required formal charge on the sulfur atom in the bisulphate molecule is \[+2\]
Hence option (B) is the correct answer.
Note: Formal charge can be defined as the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between atoms, regardless of the relative electronegativity.
The formal charge on an atom in a molecule reflects the electron count associated with the atom compared to the isolated neutral atom.
Using the above relation, find the formal charge on the sulfur atom in bisulphate ion.
Complete step by step answer:
We know that the relation to find the formal charge mathematical can be expressed as shown below:
$formal\,charge=valence\text{ }electrons-\left( non-bonding\text{ }valance\text{ }electrons \right)-\dfrac{\left( bonding\text{ }electrons \right)}{2}$
The structure of the bisulphate ion is as follows:
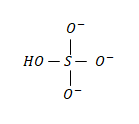
In which the middle atom is the sulfur atom.
The valence electrons of the sulfur atom is = 06 electrons;
The non-bonding valence electrons of the sulfur atom in the above molecule is = 0 electrons;
The bonding valence electrons of the sulfur atom in the above molecule is = 08 electrons.
Put all these values in the above formula and calculate the formal charge
After substituting all the values in the above relation we obtain
$formal\,charge=6-\left( 0 \right)-\dfrac{8}{2}\text{ = +2 }$
Therefore the required formal charge on the sulfur atom in the bisulphate molecule is \[+2\]
Hence option (B) is the correct answer.
Note: Formal charge can be defined as the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between atoms, regardless of the relative electronegativity.
The formal charge on an atom in a molecule reflects the electron count associated with the atom compared to the isolated neutral atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




