
In \[{\text{Br}}{{\text{F}}_{\text{3}}}\] molecule, the lone pairs occupy equatorial position to minimize:
A.Lone pair-Bond pair repulsion only
B.Bond pair-Bond pair repulsion only
C.Lone pair-Lone pair repulsion and Lone pair-Bond pair repulsion
D.Lone pair-Lone pair repulsion only
Answer
582.6k+ views
Hint: The electrons which are shared to form linkage bonds between the atoms are known as bond pairs and the pair of valence electrons which do not participate in bonding are known as lone pairs. The electrons of lone pairs are always present in pairs.
Complete step by step answer:
present between the atoms and responsible for bond formation are known as bond pairs. And the electrons which are left unshared and don't take part in any bond formation are known as lone pairs. Basically the pair of valence electrons which don't participate in bonding is known as lone pair.
When a structure of compound is made two positions play an important role in placing the outer atoms equatorial positions and axial positions. Axial bonds are the vertical one and equatorial bonds are the horizontal bonds.
The structure of the \[{\text{Br}}{{\text{F}}_{\text{3}}}\] molecule is as follows:
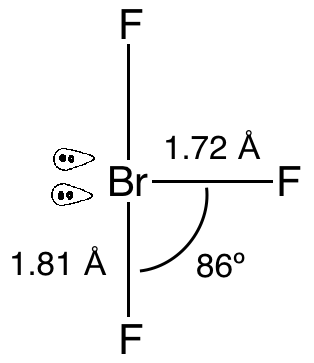
As we can see that it has two lone pairs and three bond pairs. To minimize the repulsion the lone pairs will occupy the equatorial positions as it provides them more space to expand and finally they will have minimum repulsion. Due to presence of lone pairs the angle of bonds are also affected, the bonds are tilted due to repulsion and as a result the angle decreases to $86^\circ $ from the initial angle $90^\circ $ .
So, the correct answer is Option C.
Note:
The position of atoms is decided by the hybridization of the central atom of a molecule. First the atom tries to remain in a plane and when the number of outer atoms exceeds the atoms start occupying positions in 3D plane to minimize the repulsion.
Complete step by step answer:
present between the atoms and responsible for bond formation are known as bond pairs. And the electrons which are left unshared and don't take part in any bond formation are known as lone pairs. Basically the pair of valence electrons which don't participate in bonding is known as lone pair.
When a structure of compound is made two positions play an important role in placing the outer atoms equatorial positions and axial positions. Axial bonds are the vertical one and equatorial bonds are the horizontal bonds.
The structure of the \[{\text{Br}}{{\text{F}}_{\text{3}}}\] molecule is as follows:

As we can see that it has two lone pairs and three bond pairs. To minimize the repulsion the lone pairs will occupy the equatorial positions as it provides them more space to expand and finally they will have minimum repulsion. Due to presence of lone pairs the angle of bonds are also affected, the bonds are tilted due to repulsion and as a result the angle decreases to $86^\circ $ from the initial angle $90^\circ $ .
So, the correct answer is Option C.
Note:
The position of atoms is decided by the hybridization of the central atom of a molecule. First the atom tries to remain in a plane and when the number of outer atoms exceeds the atoms start occupying positions in 3D plane to minimize the repulsion.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




