
In carbocation carbon bearing the positive charge is:
A. ${\text{s}}{{\text{p}}^{\text{2}}}$ - hybridized
B. ${\text{s}}{{\text{p}}^{\text{3}}}{\text{d}}$- hybridized
C. ${\text{sp}}$- hybridized
D. ${\text{s}}{{\text{p}}^3}$- hybridized
Answer
560.7k+ views
Hint: To answer this question we should know what carbocation is. The carbon having positive charge is known as carbocation. A carbon can form maximum four sigma bonds. The number of sigma bonds tells the hybridization. For the formation of carbocation, one bond will break, so the carbocation can have a maximum of three sigma bonds.
Complete step-by-step answer:
The carbon has four electrons in valence shell in outermost s and p-orbital. One s and three p-orbitals combine to form four hydride orbitals, so a carbon can form maximum sigma bonds. So, the hybridization of carbon having four sigma bonds is ${\text{s}}{{\text{p}}^{\text{3}}}$.
When one sigma bond of the carbon having four sigma bonds, breaks and the leaving group takes electrons along itself then a carbocation forms. Means a carbocation is formed by the removal of an electron from the carbon.
The formation of carbocation is shown as follows:
When carbon having four sigma bonds forms carbocation the hybridization changes. The change is shown as follows:
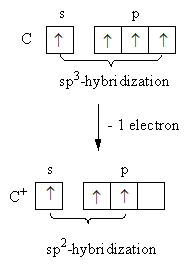
As after the removal of one electron carbon has only three electrons to share with other atoms to form a bond. So, one, s and two, p-orbitals of carbon combine to give the hybridization${\text{s}}{{\text{p}}^{\text{2}}}$. So, in carbocation carbon bearing the positive charge is ${\text{s}}{{\text{p}}^{\text{2}}}$ -hybridized.
Therefore, option (A) ${\text{s}}{{\text{p}}^{\text{2}}}$ -hybridized, is correct.
Note: Carbocation is a planer structure because the geometry related with ${\text{s}}{{\text{p}}^{\text{2}}}$ hybridization is planer. In unimolecular nucleophilic substitution carbocation forms. The carbonation is an electron deficient species. The electron donating group stabilizes the carbocation. The carbocation whose all three valencies are satisfied with three other carbon is known as tertiary carbocation. The carbocation whose two valencies are satisfied with two other carbon atoms and one is satisfied with hydrogen is known as secondary carbocation. The carbocation whose only one valency is satisfied with one other carbon atom and two are satisfied with hydrogen is known as primary carbocation.
Complete step-by-step answer:
The carbon has four electrons in valence shell in outermost s and p-orbital. One s and three p-orbitals combine to form four hydride orbitals, so a carbon can form maximum sigma bonds. So, the hybridization of carbon having four sigma bonds is ${\text{s}}{{\text{p}}^{\text{3}}}$.
When one sigma bond of the carbon having four sigma bonds, breaks and the leaving group takes electrons along itself then a carbocation forms. Means a carbocation is formed by the removal of an electron from the carbon.
The formation of carbocation is shown as follows:

When carbon having four sigma bonds forms carbocation the hybridization changes. The change is shown as follows:

As after the removal of one electron carbon has only three electrons to share with other atoms to form a bond. So, one, s and two, p-orbitals of carbon combine to give the hybridization${\text{s}}{{\text{p}}^{\text{2}}}$. So, in carbocation carbon bearing the positive charge is ${\text{s}}{{\text{p}}^{\text{2}}}$ -hybridized.
Therefore, option (A) ${\text{s}}{{\text{p}}^{\text{2}}}$ -hybridized, is correct.
Note: Carbocation is a planer structure because the geometry related with ${\text{s}}{{\text{p}}^{\text{2}}}$ hybridization is planer. In unimolecular nucleophilic substitution carbocation forms. The carbonation is an electron deficient species. The electron donating group stabilizes the carbocation. The carbocation whose all three valencies are satisfied with three other carbon is known as tertiary carbocation. The carbocation whose two valencies are satisfied with two other carbon atoms and one is satisfied with hydrogen is known as secondary carbocation. The carbocation whose only one valency is satisfied with one other carbon atom and two are satisfied with hydrogen is known as primary carbocation.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




