
In diborane, the two H-B-H angles are nearly:
\[
A.{60^0}C,{\text{ }}{120^0}C \\
B.{90^0}C,{\text{ }}{120^0}C \\
C.{95^0}C,{\text{ }}{150^0}C \\
D.{120^0}C,{\text{ }}{180^0}C \\
\]
Answer
497.1k+ views
Hint: The structure of diborane is very simple; it has $2$ boron atoms bonded to $6$ hydrogen atoms. The two boron atoms are $s{p^3}$ hybridized. There is also the presence of two bridges consisting of B-H-B. The bonds between the bridges are weak whereas between the four B-H are strong covalent bonds.
Complete answer:
The chemical formula of diborane is ${B_2}{H_6}$. It is a colorless, pyrophoric gas with a repulsively sweet odor. This compound is highly unstable at room temperature. The compounds consisting of boron and hydrogen atoms are called boranes. Diborane is one of the simplest boron hydrides.
Structure of diborane
The structure of the Diborane molecule consists of four hydrogen atoms and that of two boron atoms coming on the same plane. In between these planes, there are said to be two dividing atoms of hydrogen.
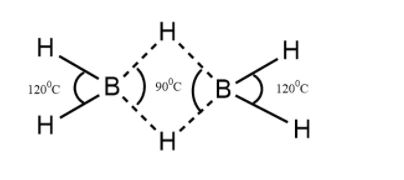
In the image, the two H-B-H angles are around \[\;{90^0}C{\text{ and }}{120^0}C\]
Therefore the correct option is \[B.\;\;\;\;\;{90^0}C,{\text{ }}{120^0}C\]
Note:
Diborane is said to be a colorless and highly flammable type of gas at room temperatures. It mixes well with air and easily forms explosive mixtures. At high concentrations, it ignites rapidly in the presence of moist air at room temperature. It releases a huge amount of energy when burnt in the presence of oxygen. Diborane readily hydrolyzes in water to give hydrogen gas and boric acid.
Complete answer:
The chemical formula of diborane is ${B_2}{H_6}$. It is a colorless, pyrophoric gas with a repulsively sweet odor. This compound is highly unstable at room temperature. The compounds consisting of boron and hydrogen atoms are called boranes. Diborane is one of the simplest boron hydrides.
Structure of diborane
The structure of the Diborane molecule consists of four hydrogen atoms and that of two boron atoms coming on the same plane. In between these planes, there are said to be two dividing atoms of hydrogen.

In the image, the two H-B-H angles are around \[\;{90^0}C{\text{ and }}{120^0}C\]
Therefore the correct option is \[B.\;\;\;\;\;{90^0}C,{\text{ }}{120^0}C\]
Note:
Diborane is said to be a colorless and highly flammable type of gas at room temperatures. It mixes well with air and easily forms explosive mixtures. At high concentrations, it ignites rapidly in the presence of moist air at room temperature. It releases a huge amount of energy when burnt in the presence of oxygen. Diborane readily hydrolyzes in water to give hydrogen gas and boric acid.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




