
In Dow’s process for manufacture of phenol, PhCl is fused with NaOH at elevated temperature under pressure.
\[PhCl\xrightarrow[{623K,300atm}]{{NaOH}}[Intermediate]\xrightarrow{{{H_2}O}}Phenol(A) + (B + C)sideproduct\]
Which of the following statements are correct?
(This question has multiple correct options)
(A) Phenol is formed via the formation of benzyne intermediate
(B) p – phenyl phenol is also formed as a by product
(C) Diphenyl ether is also formed as a by – product
(D) Biphenylene is also formed as a by – product
Answer
566.7k+ views
Hint: Dow’s process is hydrolysis of chlorobenzene. It is a nucleophilic substitution reaction. It is formed by an intermediate of benzene. Nucleophilic substitution is a type of reaction in which an electron rich compound replaces a leaving group.
Complete answer:
- We refer to Dow's Process to the hydrolysis of chlorobenzene for the preparation of phenol.
-We can easily convert benzene chlorobenzene by electrophilic aromatic substitution.
- Dow’s process is preparation of phenol from haloarenes, it is an industrial preparation of phenol.
- Chlorobenzenes with 10% NaOH solution is heated at 623K under 300 atm pressure.
- The major product of Dow’s process is phenol but other by- products are also formed as a result of reaction between the species formed during the reaction.
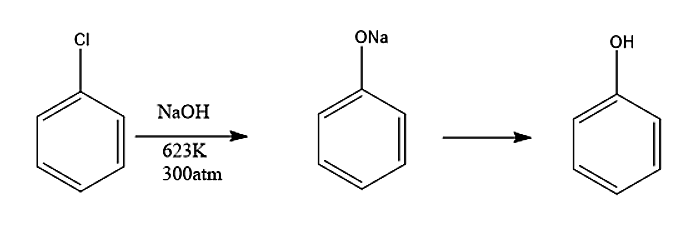
- Now let’s understand the reaction mechanism:
-In the first step, the base (NaOH) removes one hydrogen from the benzene ring and forms a carbanion which loses \[C{l^ - }\] ion to form benzyne intermediate.
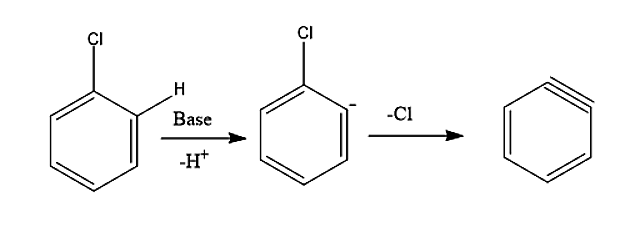
-In the second step, an attack of nucleophile takes place. The benzyne intermediate formed gets attacked by nucleophile \[O{H^ - }\]to form carbanion which subsequently gets protonated to form phenol.
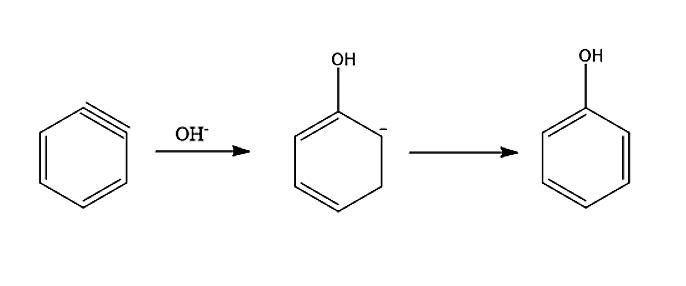
- Now for side products, let’s see the conversion of reactants into reacting species.
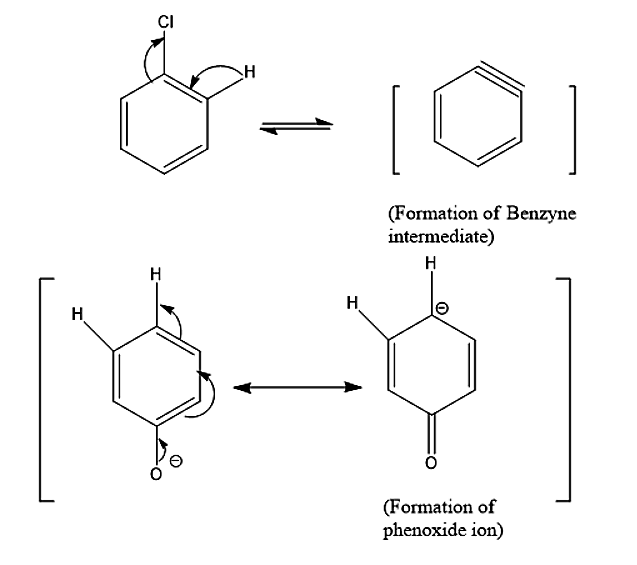
- Due to the presence of phenoxide ions diphenyl ether is also a by – product.
- Another by – product we see is para- phenyl phenol which is formed by benzyne and phenoxide ions.
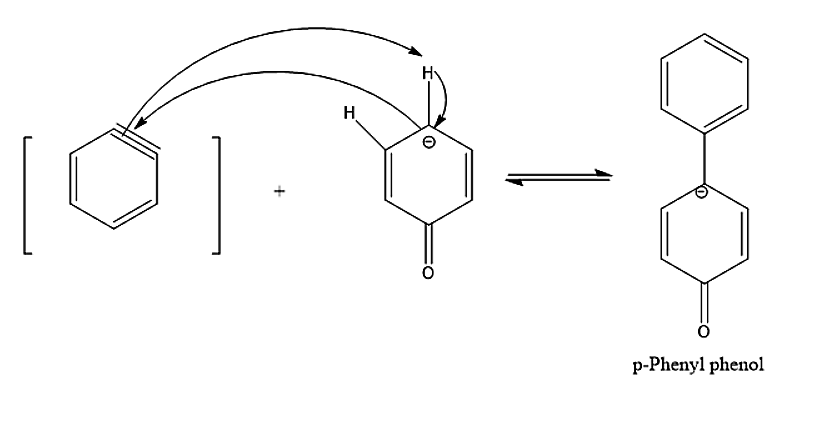
Therefore, the correct statements are options A. Phenol is formed via the formation of benzyne intermediate, B. p – phenyl phenol is also formed as a by-product, C. Diphenyl ether is also formed as a by – product.
Note:
-After the formation of benzyne, \[O{H^ - }\] nucleophilic attack takes place.
-The reaction mixture also contains some phenoxide ions. So, Diphenyl ether is a possible side product.
- The major product of Dow’s process is phenol but other by- products are also formed as a result of reaction between the species formed during the reaction.
Complete answer:
- We refer to Dow's Process to the hydrolysis of chlorobenzene for the preparation of phenol.
-We can easily convert benzene chlorobenzene by electrophilic aromatic substitution.
- Dow’s process is preparation of phenol from haloarenes, it is an industrial preparation of phenol.
- Chlorobenzenes with 10% NaOH solution is heated at 623K under 300 atm pressure.
- The major product of Dow’s process is phenol but other by- products are also formed as a result of reaction between the species formed during the reaction.

- Now let’s understand the reaction mechanism:
-In the first step, the base (NaOH) removes one hydrogen from the benzene ring and forms a carbanion which loses \[C{l^ - }\] ion to form benzyne intermediate.

-In the second step, an attack of nucleophile takes place. The benzyne intermediate formed gets attacked by nucleophile \[O{H^ - }\]to form carbanion which subsequently gets protonated to form phenol.

- Now for side products, let’s see the conversion of reactants into reacting species.

- Due to the presence of phenoxide ions diphenyl ether is also a by – product.
- Another by – product we see is para- phenyl phenol which is formed by benzyne and phenoxide ions.

Therefore, the correct statements are options A. Phenol is formed via the formation of benzyne intermediate, B. p – phenyl phenol is also formed as a by-product, C. Diphenyl ether is also formed as a by – product.
Note:
-After the formation of benzyne, \[O{H^ - }\] nucleophilic attack takes place.
-The reaction mixture also contains some phenoxide ions. So, Diphenyl ether is a possible side product.
- The major product of Dow’s process is phenol but other by- products are also formed as a result of reaction between the species formed during the reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




