
In hydrofluorosilicic acid, the covalency of $ Si $ is
A. $ 2 $
B. $ 4 $
C. $ 6 $
D. $ 8 $
Answer
511.8k+ views
Hint :Mutual sharing of electrons between atoms to form stable compounds is known as covalent bonding. The maximum number of bonds an atom can form to gain stability in its uncharged state is known as its covalency. For example, in ammonia molecule i.e., $ N{H_3} $ , nitrogen atom form three bonds with hydrogen atom means the number of share electrons is $ 3 $ , so the covalency of nitrogen in $ N{H_3} $ is $ 3 $ .
Complete Step By Step Answer:
Hydrofluorosilicic acid also known as hexafluorosilicic acid is an inorganic compound with chemical formula $ {\left( {{H_3}O} \right)_2}Si{F_6} $ . In the structure of hydrofluorosilicic acid, it consists of hydronium ions i.e., $ {H_3}{O^ + } $ which are balanced by hexafluorosilicate dianions. In aqueous solution, hydronium cation is represented with a solvated proton and hence the formula is represented as $ {H_2}Si{F_6} $ .
Structurally, hydrofluorosilicic acid is represented as follows:
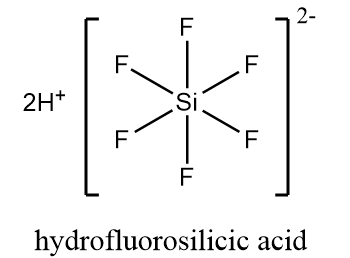
In hexafluorosilicate dianion, silicon consists of six electrons in its valence shell which are shared by six fluoride ions to form $ SiF_6^{2 - } $ ion. The ion is $ {d^2}s{p^3} $ hybridized and contains no lone pair of electrons and six bonding pairs of electrons around the silicon atom arranged in an octahedral geometry.
So, number of electron pair shared by silicon $ = 6 $
Hence, the covalency of silicon ( $ Si $ ) in hydrofluorosilicic acid $ = 6 $ .
Thus, option (C) is the correct answer.
Note :
It is important to note that although carbon and silicon belong from the same group but the maximum covalency shown by carbon atom is four whereas the maximum covalency shown by silicon atom is six because of presence of vacant d-orbitals in silicon, it can share all its valence electrons to form covalent bond.
Complete Step By Step Answer:
Hydrofluorosilicic acid also known as hexafluorosilicic acid is an inorganic compound with chemical formula $ {\left( {{H_3}O} \right)_2}Si{F_6} $ . In the structure of hydrofluorosilicic acid, it consists of hydronium ions i.e., $ {H_3}{O^ + } $ which are balanced by hexafluorosilicate dianions. In aqueous solution, hydronium cation is represented with a solvated proton and hence the formula is represented as $ {H_2}Si{F_6} $ .
Structurally, hydrofluorosilicic acid is represented as follows:

In hexafluorosilicate dianion, silicon consists of six electrons in its valence shell which are shared by six fluoride ions to form $ SiF_6^{2 - } $ ion. The ion is $ {d^2}s{p^3} $ hybridized and contains no lone pair of electrons and six bonding pairs of electrons around the silicon atom arranged in an octahedral geometry.
So, number of electron pair shared by silicon $ = 6 $
Hence, the covalency of silicon ( $ Si $ ) in hydrofluorosilicic acid $ = 6 $ .
Thus, option (C) is the correct answer.
Note :
It is important to note that although carbon and silicon belong from the same group but the maximum covalency shown by carbon atom is four whereas the maximum covalency shown by silicon atom is six because of presence of vacant d-orbitals in silicon, it can share all its valence electrons to form covalent bond.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




