
In Liebermann’s nitroso reaction, change in colour of phenol occurs as:
A. Brown or red-greenish red-deep blue
B. Red-deep blue-green
C. Red-green-white
D. White-red-green
Answer
570k+ views
Hint: The answer to this question is based on the fact that Liebermann’s test is carried out for the presence of nitroso group and that includes treating the phenol with sodium nitrate and sulphuric acid produces one coloured compound and then diluting it where colour changes followed by treating with base that gives original colour.
Complete step by step answer:
In the lower classes of organic chemistry, we have studied about the various laboratory tests used for the detection of functional groups.
Now, let us see what is Liebermann’s test used for and which functional group the test confirms.
- Liebermann’s test is basically used for the detection or identification of presence of the nitroso functional group. Particularly, this test is used for the testing of secondary amines and thus the reaction undergoing when phenol is subjected to this test is as said below.
- When phenol is treated with sodium nitrate $(NaN{{O}_{2}})$ and concentrated sulphuric acid $({{H}_{2}}S{{O}_{4}})$, the product formed will have deep green or blue colour which when diluted with water, it changes to red.
- This diluted product when treated with a base like $NaOH/KOH$, the original colour is restored and thus this reaction is termed as Liebermann’s nitroso test. the schematic reaction is shown below,
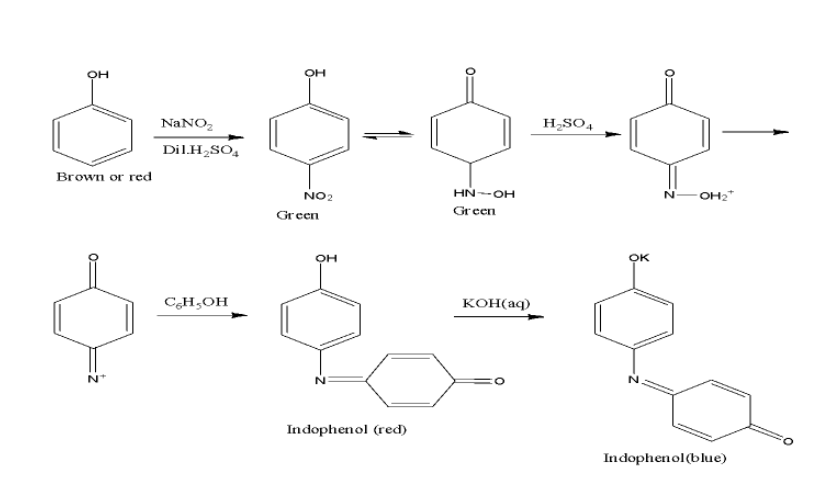
Note: The Liebermann’s test is also known as Liebermann – Burchard or anhydride test that is also used for the detection of cholesterol. The formation of green or green – blue colour after a few minutes confirms the presence of cholesterol. This reagent that is Liebermann - Burchard reagent is used in a colorimetric test to detect cholesterol.
Complete step by step answer:
In the lower classes of organic chemistry, we have studied about the various laboratory tests used for the detection of functional groups.
Now, let us see what is Liebermann’s test used for and which functional group the test confirms.
- Liebermann’s test is basically used for the detection or identification of presence of the nitroso functional group. Particularly, this test is used for the testing of secondary amines and thus the reaction undergoing when phenol is subjected to this test is as said below.
- When phenol is treated with sodium nitrate $(NaN{{O}_{2}})$ and concentrated sulphuric acid $({{H}_{2}}S{{O}_{4}})$, the product formed will have deep green or blue colour which when diluted with water, it changes to red.
- This diluted product when treated with a base like $NaOH/KOH$, the original colour is restored and thus this reaction is termed as Liebermann’s nitroso test. the schematic reaction is shown below,

Note: The Liebermann’s test is also known as Liebermann – Burchard or anhydride test that is also used for the detection of cholesterol. The formation of green or green – blue colour after a few minutes confirms the presence of cholesterol. This reagent that is Liebermann - Burchard reagent is used in a colorimetric test to detect cholesterol.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




