
In ${{N}_{2}}{{H}_{4}}$ molecule type of overlapping present between N-H bond:-
(A) $s-p$
(B) $s{{p}^{2}}-p$
(C) $s{{p}^{3}}-s$
(D) $s{{p}^{2}}-s$
Answer
582.9k+ views
Hint: The molecule ${{N}_{2}}{{H}_{4}}$ is called hydrazine. From drawing the Lewis structure of ${{N}_{2}}{{H}_{4}}$, we could identify that each nitrogen atom is $s{{p}^{3}}$ hybridized and uses two $s{{p}^{3}}$ orbitals to form N–H bonds and hydrogen has only one electron in its 1s orbital.
Complete step by step solution:
- Hydrazine is a colourless liquid with an ammoniacal odour and the hydrazine is miscible with water in all proportions. Also, its aqueous solutions are highly alkaline in nature.
- In order to get an idea of overlapping present between N-H bonds in ${{N}_{2}}{{H}_{4}}$ molecules, we need to look at the concept of hybridization. It is the process in which the overlap of bonding orbitals takes place and as a result, the formation of stronger bonds occur. Using the model of hybridization we would be able to predict the shapes of certain molecules.
- In hybridization, the atomic orbitals which have similar energy but not equivalent are combined mathematically in such a way to produce sets of equivalent orbitals which are properly oriented to form bonds. Since they are produced by hybridizing two or more atomic orbitals from the same atom, these new combinations are called hybrid atomic orbitals.
- The Lewis structure of ${{N}_{2}}{{H}_{4}}$ molecule is given below
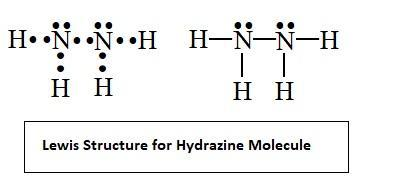
- From the Lewis structure it’s clear that every nitrogen atom has one lone pair of electrons and is bonded to three other atoms including two hydrogens and one nitrogen atom.
- As we know the Steric Number is the sum of the number of lone electron pairs on the central atom and number of atoms bonded to the central atom. So the steric number for nitrogen is $4(3+1)$. This implies that four hybrid orbitals are needed by every nitrogen atom and this is possible only through $s{{p}^{3}}$ hybridization.
- Since hydrogen has only one s orbital, it has an s overlapping. Thus the overlapping present between N-H bonds in hydrazine will be $s{{p}^{3}}-s$.
Thus the answer is option (C). $s{{p}^{3}}-s$.
Note: It should be noted that the bond between nitrogen atoms in hydrazine is single, not a triple bond. It’s because we only have one p-orbital per nitrogen atom that is available to form a pi-bond, and in order to form two pi-bonds, we need at least two p-orbitals per each nitrogen atom to be available as in the case of acetylene.
Complete step by step solution:
- Hydrazine is a colourless liquid with an ammoniacal odour and the hydrazine is miscible with water in all proportions. Also, its aqueous solutions are highly alkaline in nature.
- In order to get an idea of overlapping present between N-H bonds in ${{N}_{2}}{{H}_{4}}$ molecules, we need to look at the concept of hybridization. It is the process in which the overlap of bonding orbitals takes place and as a result, the formation of stronger bonds occur. Using the model of hybridization we would be able to predict the shapes of certain molecules.
- In hybridization, the atomic orbitals which have similar energy but not equivalent are combined mathematically in such a way to produce sets of equivalent orbitals which are properly oriented to form bonds. Since they are produced by hybridizing two or more atomic orbitals from the same atom, these new combinations are called hybrid atomic orbitals.
- The Lewis structure of ${{N}_{2}}{{H}_{4}}$ molecule is given below

- From the Lewis structure it’s clear that every nitrogen atom has one lone pair of electrons and is bonded to three other atoms including two hydrogens and one nitrogen atom.
- As we know the Steric Number is the sum of the number of lone electron pairs on the central atom and number of atoms bonded to the central atom. So the steric number for nitrogen is $4(3+1)$. This implies that four hybrid orbitals are needed by every nitrogen atom and this is possible only through $s{{p}^{3}}$ hybridization.
- Since hydrogen has only one s orbital, it has an s overlapping. Thus the overlapping present between N-H bonds in hydrazine will be $s{{p}^{3}}-s$.
Thus the answer is option (C). $s{{p}^{3}}-s$.
Note: It should be noted that the bond between nitrogen atoms in hydrazine is single, not a triple bond. It’s because we only have one p-orbital per nitrogen atom that is available to form a pi-bond, and in order to form two pi-bonds, we need at least two p-orbitals per each nitrogen atom to be available as in the case of acetylene.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




