
In photoelectric effect, the number of photoelectrons emitted is proportional to
A) velocity of incident beam
B) frequency of incident beam
C) intensity of incident beam
D) work function of cathode material.
Answer
586.8k+ views
Hint:The photoelectric effect is the phenomenon in which some materials emit electrons in the presence of light.
Hallwach Lenard’s experiment on photoelectric effect successfully helped in confirming Einstein's theory of photoelectric effect.
Complete step-by-step answer:
Here is the experimental setup for the photoelectric experiment for the study of photoelectric effect:
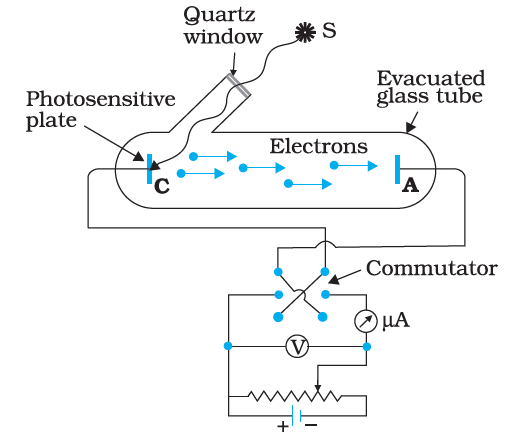
The experimental setup consists of an evacuated glass tube in which there are two electrodes. There is a quartz window which allows the light source through the glass tube. The electrodes are connected to a commutator which is used for changing the direction of the electric current.
One such experiment performed is the effect of intensity on photocurrent –
The collector anode is maintained at a constant positive potential with respect to cathode C which emits electrons. The light window is opened, and the ammeter reading is recorded. Now, keeping the frequency and potential fixed, the intensity of the incident radiation is increased slowly. We see that the current increases linearly with the increase in the intensity.
Thus, the number of photoelectrons emitted per second is directly proportional to the intensity of the light.
Hence, the correct option is Option C.
Note: The premise for classifying the light consists of particle is primarily, based on the Planck’s Law, which states that –
Energy, $E \propto \upsilon $
$E = h\upsilon $
A photon is defined as a particle whose energy is equal to $E = h\upsilon $. The photoelectric effect proves the theory of light as a particle.
Hallwach Lenard’s experiment on photoelectric effect successfully helped in confirming Einstein's theory of photoelectric effect.
Complete step-by-step answer:
Here is the experimental setup for the photoelectric experiment for the study of photoelectric effect:

The experimental setup consists of an evacuated glass tube in which there are two electrodes. There is a quartz window which allows the light source through the glass tube. The electrodes are connected to a commutator which is used for changing the direction of the electric current.
One such experiment performed is the effect of intensity on photocurrent –
The collector anode is maintained at a constant positive potential with respect to cathode C which emits electrons. The light window is opened, and the ammeter reading is recorded. Now, keeping the frequency and potential fixed, the intensity of the incident radiation is increased slowly. We see that the current increases linearly with the increase in the intensity.
Thus, the number of photoelectrons emitted per second is directly proportional to the intensity of the light.
Hence, the correct option is Option C.
Note: The premise for classifying the light consists of particle is primarily, based on the Planck’s Law, which states that –
Energy, $E \propto \upsilon $
$E = h\upsilon $
A photon is defined as a particle whose energy is equal to $E = h\upsilon $. The photoelectric effect proves the theory of light as a particle.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




