
In presence of an enzyme, the activation energy
A)Increases
B)Decreases
C)First increases and then decreases
D)Activation energy is not affected at all
Answer
574.2k+ views
Hint: When the energy required to initiate the reaction this is known as the activation energy (${ E }_{ A }$). IT is the amount of energy required for the reactions to reach the transition state. The source of energy that is needed to push the reactions in a forward direction is heat energy taken from the surroundings.
Complete answer:
-A catalyst is a substance that speeds up a chemical reaction without being a reactant. Enzymes are the catalyst needed in the biochemical reactions that generally happen in living organisms. Enzymes are generally proteins but some of the molecules of ribonucleic acid also act as enzymes.
-In biology such as biochemistry, the energy of activation concerns the energy which is needed to initiate a reaction. Like - For the breakdown of glucose into pyruvic acid in the respiration process the activation energy which is required is 2 ATP.
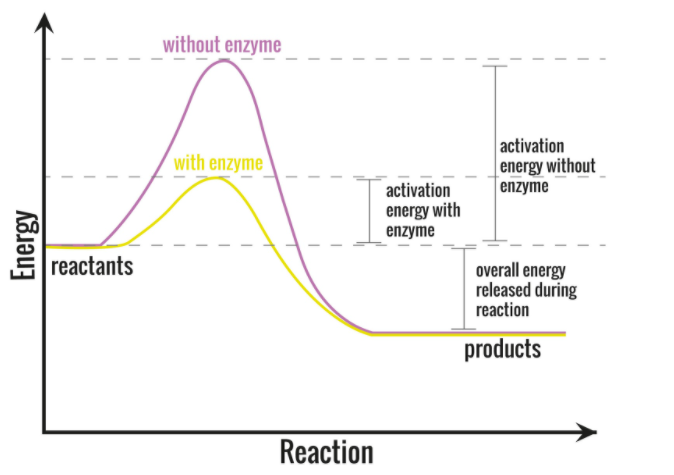
-Enzymes perform a very important task by lowering the activation energy of the reaction (the energy put in for the reaction to begin). Enzymes work in a way that it binds to the reactant molecule and holds in such a way that breaking of the chemical bond and forming of the bond processes takes place more fluently.
-This lowering of energy allows the biological reaction to proceed very quickly at a very low temperature which is bearable by living organisms.
Additional Information: -In a particular reaction the activation energy determines the rate at which the reaction will proceed. If the activation energy is higher than the chemical reaction will be slow. For example, the conversion of diamond into graphite takes a very long time, the same with the rusting of iron. The reaction in them occurs very slow because of their high activation energy barriers.
-The macromolecules like proteins, DNA, and RNA have a considerable amount of energy and their breakdown is exergonic. When a breakdown of these molecules takes place, enough heat is provided by their cellular temperature for the exergonic reactions to control their energy activation barriers. This led to the disintegration of the important component of a cell. So important cellular reactions to occur at appreciable rates the activation energy must be lowered and for this, enzymes are very useful.
So, the correct answer is,” decreases”
Note: -Enzymes bind to one or more reactant molecules to catalyze a reaction. These molecules refer to the enzyme-substrate.
-The substrates bind to the part of the enzymes known as the active sites. This led to catalytic action to start.
-The temperature and pH may affect the active site and function of the enzyme.
Complete answer:
-A catalyst is a substance that speeds up a chemical reaction without being a reactant. Enzymes are the catalyst needed in the biochemical reactions that generally happen in living organisms. Enzymes are generally proteins but some of the molecules of ribonucleic acid also act as enzymes.
-In biology such as biochemistry, the energy of activation concerns the energy which is needed to initiate a reaction. Like - For the breakdown of glucose into pyruvic acid in the respiration process the activation energy which is required is 2 ATP.
-Enzymes perform a very important task by lowering the activation energy of the reaction (the energy put in for the reaction to begin). Enzymes work in a way that it binds to the reactant molecule and holds in such a way that breaking of the chemical bond and forming of the bond processes takes place more fluently.
-This lowering of energy allows the biological reaction to proceed very quickly at a very low temperature which is bearable by living organisms.
Additional Information: -In a particular reaction the activation energy determines the rate at which the reaction will proceed. If the activation energy is higher than the chemical reaction will be slow. For example, the conversion of diamond into graphite takes a very long time, the same with the rusting of iron. The reaction in them occurs very slow because of their high activation energy barriers.
-The macromolecules like proteins, DNA, and RNA have a considerable amount of energy and their breakdown is exergonic. When a breakdown of these molecules takes place, enough heat is provided by their cellular temperature for the exergonic reactions to control their energy activation barriers. This led to the disintegration of the important component of a cell. So important cellular reactions to occur at appreciable rates the activation energy must be lowered and for this, enzymes are very useful.
So, the correct answer is,” decreases”
Note: -Enzymes bind to one or more reactant molecules to catalyze a reaction. These molecules refer to the enzyme-substrate.
-The substrates bind to the part of the enzymes known as the active sites. This led to catalytic action to start.
-The temperature and pH may affect the active site and function of the enzyme.

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




