
In terms of period and group where would you locate the elements with Z= 114?
Answer
583.2k+ views
Hint: Periodic made the study of elements and their properties easy. Periodic table made the study of the chemistry of elements. The elements in the modern periodic table are arranged on the basis of atomic number.
Complete step by step answer:
The periodic table was discovered by Dmitri Mendeleev in 1869. Periodic table contains 118 elements. Mendeleev law states that "The properties of the elements are the periodic function of their atomic number", so elements in the periodic table are arranged on the basis of atomic number.We know that there are in total 7 periods and 18 groups. Periodic tables made the study of elements and their properties easy. The factor that made it easy to study properties of the elements that down the group, the properties of elements follow a trend. Same goes to elements of a particular period.
First, let’s see the representation atom structure in the periodic table.
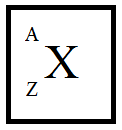
Where, X is the element
A is the mass number
Z is the atomic number
In question it is given that Z=114, that is atomic number is 114. It is very important to remember atomic numbers of noble gases. The atomic number of noble gases are:
He = 2
Ne = 10
Ar = 18
Kr = 36
Xe = 54
Rn = 86
Og = 118
We know that noble gases belong to the group 18. If we move 4 groups left from group 18, we will arrive at the group where the element with Z=114 is located. The nearest noble gas to the element whose atomic number 114 is oganesson (Og). Og belongs to ${7^{th}}$period so, the element with atomic number 114 belongs to ${7^{th}}$ period as the atomic number increases from left to right across the group.
Thus, from the above discussion we can conclude that the element with atomic number 114 is the ${7^{th}}$ period and ${15^{th}}$ group.
Note: It is very important to remember the atomic number of the noble gas to solve this type of question and it also used to write electronic configuration. Atomic number increases on moving from left to right across the group.
Complete step by step answer:
The periodic table was discovered by Dmitri Mendeleev in 1869. Periodic table contains 118 elements. Mendeleev law states that "The properties of the elements are the periodic function of their atomic number", so elements in the periodic table are arranged on the basis of atomic number.We know that there are in total 7 periods and 18 groups. Periodic tables made the study of elements and their properties easy. The factor that made it easy to study properties of the elements that down the group, the properties of elements follow a trend. Same goes to elements of a particular period.
First, let’s see the representation atom structure in the periodic table.
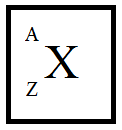
Where, X is the element
A is the mass number
Z is the atomic number
In question it is given that Z=114, that is atomic number is 114. It is very important to remember atomic numbers of noble gases. The atomic number of noble gases are:
He = 2
Ne = 10
Ar = 18
Kr = 36
Xe = 54
Rn = 86
Og = 118
We know that noble gases belong to the group 18. If we move 4 groups left from group 18, we will arrive at the group where the element with Z=114 is located. The nearest noble gas to the element whose atomic number 114 is oganesson (Og). Og belongs to ${7^{th}}$period so, the element with atomic number 114 belongs to ${7^{th}}$ period as the atomic number increases from left to right across the group.
Thus, from the above discussion we can conclude that the element with atomic number 114 is the ${7^{th}}$ period and ${15^{th}}$ group.
Note: It is very important to remember the atomic number of the noble gas to solve this type of question and it also used to write electronic configuration. Atomic number increases on moving from left to right across the group.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




