
In the electronic structure of ${H_2}S{O_4}$ the total number of unshared electrons is:
A. $20$
B. $16$
C. $12$
D. $8$
Answer
570.6k+ views
Hint: The total number of unshared electrons in a molecule is the sum of the lone pairs of electrons in each atom of the molecule. Thus, by drawing the structure of the molecule, we can find out the number of lone pairs.
Complete step by step answer:
Let us first look at the basic structure of sulphuric acid:
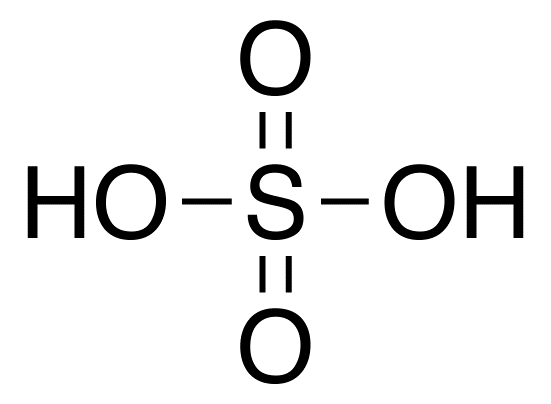
As we know, unshared pairs of electrons are the lone pairs present on each atom. Let us find out how many lone pairs are present in this molecule:
As we know, sulphur has six electrons in its valence shell, since its electronic configuration is $[Ne]3{s^2}3{p^4}$. We see that the total number of bonds formed by sulphur in this molecule is also six (two single bonds and two double bonds). As we know, in a covalent bond, each electron contributes to one bond. Hence, all electrons in sulphur are used for bonding and there are no lone pairs.
We have four oxygen atoms present. Two of them are involved in double bonds while the other two in single bonds. The electronic configuration of oxygen is $[He]2{s^2}2{p^4}$ and thus, it has six valence electrons. A double bond takes up two of these electrons for bonding. Thus, in the oxygens involved in the double bonds, there are four unshared electrons, which make up to two lone pairs. Similarly, a single bond takes up one electron. The two single bonded oxygen atoms are bonded to sulphur and hydrogen and hence, these take up two electrons each. Therefore, in the single bonded oxygens too, there are two lone pairs. Thus, each single bonded and double bonded oxygen atom has four unshared electrons, making the total number of unshared electrons coming from the oxygen atoms as:
$(4 \times 2) + (4 \times 2) = 16$
Hydrogen has just one electron in its valence shell, and these are used in making the single bonds with the four oxygens present in the molecule. Thus, it does not possess any unshared electrons.
Hence, as we can see, the unshared electrons come only from the oxygen atoms, and the total number of unshared electrons in the molecule is thus $16$.
So, the correct answer is Option B .
Note: The electronic configuration of an atom denotes the order in which electrons fill up in that particular atom. Note that sulphur has the property of forming more than six bonds, as it possesses an empty d-orbital. This ability is known as “expansion of octet”.
Complete step by step answer:
Let us first look at the basic structure of sulphuric acid:

As we know, unshared pairs of electrons are the lone pairs present on each atom. Let us find out how many lone pairs are present in this molecule:
As we know, sulphur has six electrons in its valence shell, since its electronic configuration is $[Ne]3{s^2}3{p^4}$. We see that the total number of bonds formed by sulphur in this molecule is also six (two single bonds and two double bonds). As we know, in a covalent bond, each electron contributes to one bond. Hence, all electrons in sulphur are used for bonding and there are no lone pairs.
We have four oxygen atoms present. Two of them are involved in double bonds while the other two in single bonds. The electronic configuration of oxygen is $[He]2{s^2}2{p^4}$ and thus, it has six valence electrons. A double bond takes up two of these electrons for bonding. Thus, in the oxygens involved in the double bonds, there are four unshared electrons, which make up to two lone pairs. Similarly, a single bond takes up one electron. The two single bonded oxygen atoms are bonded to sulphur and hydrogen and hence, these take up two electrons each. Therefore, in the single bonded oxygens too, there are two lone pairs. Thus, each single bonded and double bonded oxygen atom has four unshared electrons, making the total number of unshared electrons coming from the oxygen atoms as:
$(4 \times 2) + (4 \times 2) = 16$
Hydrogen has just one electron in its valence shell, and these are used in making the single bonds with the four oxygens present in the molecule. Thus, it does not possess any unshared electrons.
Hence, as we can see, the unshared electrons come only from the oxygen atoms, and the total number of unshared electrons in the molecule is thus $16$.
So, the correct answer is Option B .
Note: The electronic configuration of an atom denotes the order in which electrons fill up in that particular atom. Note that sulphur has the property of forming more than six bonds, as it possesses an empty d-orbital. This ability is known as “expansion of octet”.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




