
In the following reaction,
$Aldehyde+Alcohol\xrightarrow{HCl}Acetal$
Aldehyde Alcohol HCHO t-BuOH $C{{H}_{3}}CHO$ MeOH
Predict the best combinations from the above given aldehydes and alcohols:
A) HCHO and MeOH
B) HCHO and t-BuOH
C) $C{{H}_{3}}CHO$ and MeOH
D) $C{{H}_{3}}CHO$and t-BuOH
| Aldehyde | Alcohol |
| HCHO | t-BuOH |
| $C{{H}_{3}}CHO$ | MeOH |
Answer
568.8k+ views
Hint: For the acetal formation aldehyde acts as the electrophile and alcohol acts as the nucleophile.Inductive effect plays a major role in the reactivity of a compound.
Complete answer:
In this question, a reaction is given in which an aldehyde and an alcohol combines and forms an acetal.
We are also provided with two aldehydes-formaldehyde and acetaldehyde and two alcohols- tertiary butanol and methanol. We have to say the best combination of the aldehyde and alcohol given in the table for the formation of acetal.
So we know that for the formation of acetal and for reaction to happen there should be an electrophile and nucleophile. And to know the best combinations of aldehyde and alcohol we should know the mechanism of acetal formation.
When an alcohol reacts with aldehyde, the acidic hydrogen atom of alcohol (-OH) attacks the carbonyl oxygen atom resulting in breaking of the double bond of aldehyde and the remaining alkoxide group of the alcohol attacks the carbon by nucleophilic attack. So here aldehyde acts as the electrophile and the alcohol as the nucleophile.
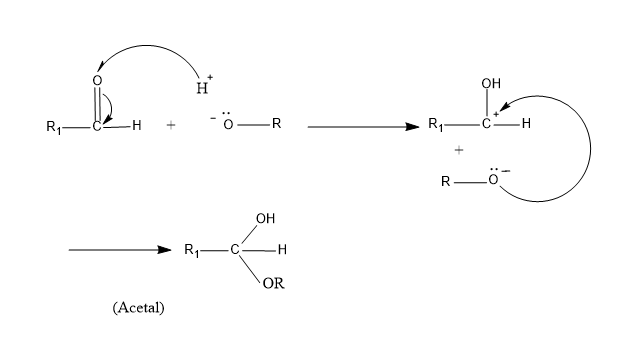
Now we have to find the best combination of aldehyde and alcohol for the effective reaction of acetal formation.
First, we have to select the aldehyde for the reaction, here formaldehyde and acetaldehyde is given as the options. First let’s see the structure of these two aldehydes.
Here if we compare these both aldehyde, acetaldehyde is having a methyl group and the methyl group is having +I effect i.e. positive inductive effect and it donates electrons to the carbonyl carbon and it makes the C less electrophilic. Hence acetaldehyde will be less electrophilic than formaldehyde as there is no methyl group in formaldehyde to possess +I effect.So from the two options, HCHO is the best option.
Now let’s move on to alcohol.
Here if we compare the structure, as alcohol acts as the nucleophile, it should have more nucleophilic nature. Here t-BuOH is having three methyl group and hence it is more nucleophilic than MeOH, but
t-BuOH will not attack the aldehyde effectively due to steric hindrance as the tertiary alkyl group is a bulky group.
Hence here MeOH will attack with the aldehyde effectively.
Hence the best combination for the acetal formation is HCHO and MeOH.
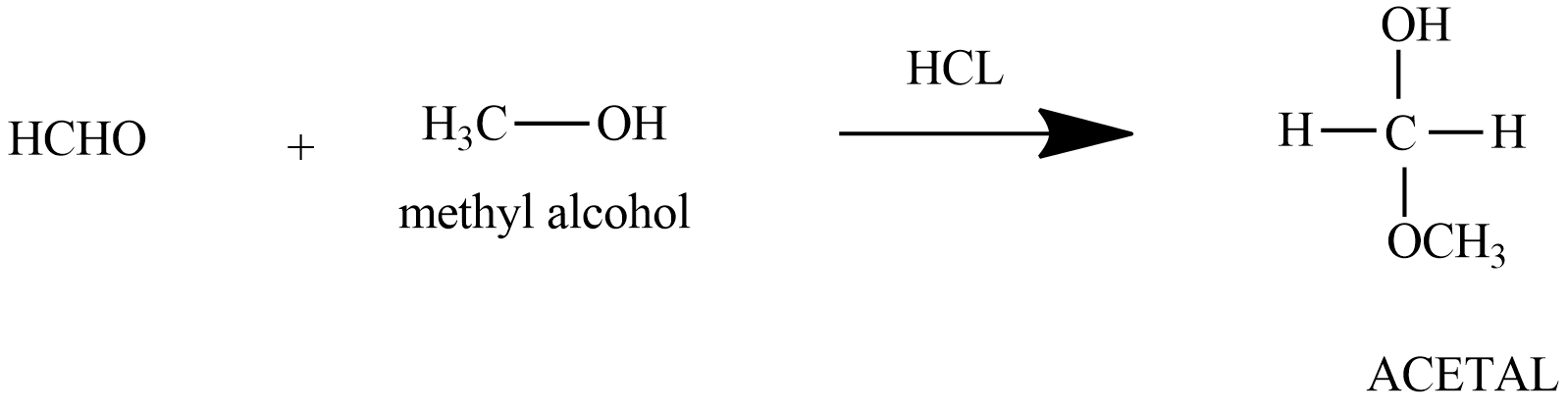
The correct option from the given combination is option (A).
Note:
An electrophile is a species which consists of positive charge on it resulting from lack of electrons on carbon atoms and nucleophile is the species with negative charge and it seeks an electrophile to form a stable compound.
Complete answer:
In this question, a reaction is given in which an aldehyde and an alcohol combines and forms an acetal.
We are also provided with two aldehydes-formaldehyde and acetaldehyde and two alcohols- tertiary butanol and methanol. We have to say the best combination of the aldehyde and alcohol given in the table for the formation of acetal.
So we know that for the formation of acetal and for reaction to happen there should be an electrophile and nucleophile. And to know the best combinations of aldehyde and alcohol we should know the mechanism of acetal formation.
When an alcohol reacts with aldehyde, the acidic hydrogen atom of alcohol (-OH) attacks the carbonyl oxygen atom resulting in breaking of the double bond of aldehyde and the remaining alkoxide group of the alcohol attacks the carbon by nucleophilic attack. So here aldehyde acts as the electrophile and the alcohol as the nucleophile.

Now we have to find the best combination of aldehyde and alcohol for the effective reaction of acetal formation.
First, we have to select the aldehyde for the reaction, here formaldehyde and acetaldehyde is given as the options. First let’s see the structure of these two aldehydes.
Formaldehyde 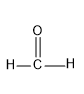
| Acetaldehyde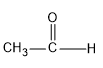
|
Here if we compare these both aldehyde, acetaldehyde is having a methyl group and the methyl group is having +I effect i.e. positive inductive effect and it donates electrons to the carbonyl carbon and it makes the C less electrophilic. Hence acetaldehyde will be less electrophilic than formaldehyde as there is no methyl group in formaldehyde to possess +I effect.So from the two options, HCHO is the best option.
Now let’s move on to alcohol.
t-BuOH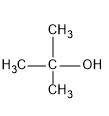
| MeOH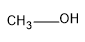
|
Here if we compare the structure, as alcohol acts as the nucleophile, it should have more nucleophilic nature. Here t-BuOH is having three methyl group and hence it is more nucleophilic than MeOH, but
t-BuOH will not attack the aldehyde effectively due to steric hindrance as the tertiary alkyl group is a bulky group.
Hence here MeOH will attack with the aldehyde effectively.
Hence the best combination for the acetal formation is HCHO and MeOH.
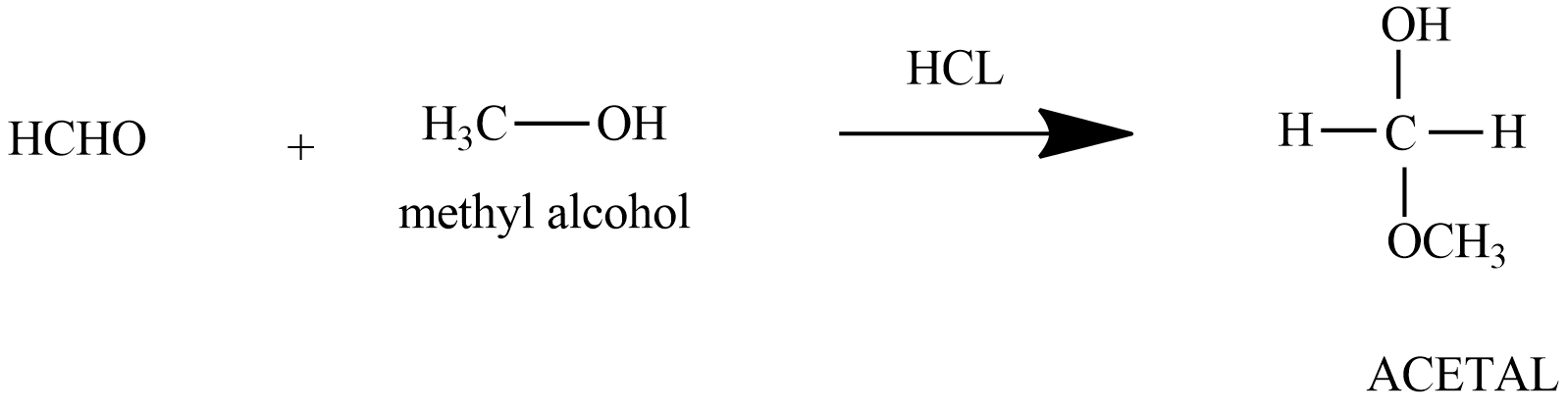
The correct option from the given combination is option (A).
Note:
An electrophile is a species which consists of positive charge on it resulting from lack of electrons on carbon atoms and nucleophile is the species with negative charge and it seeks an electrophile to form a stable compound.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




