
In the following schematic diagram for the preparation of hydrogen gas, what happen if following changes are made?
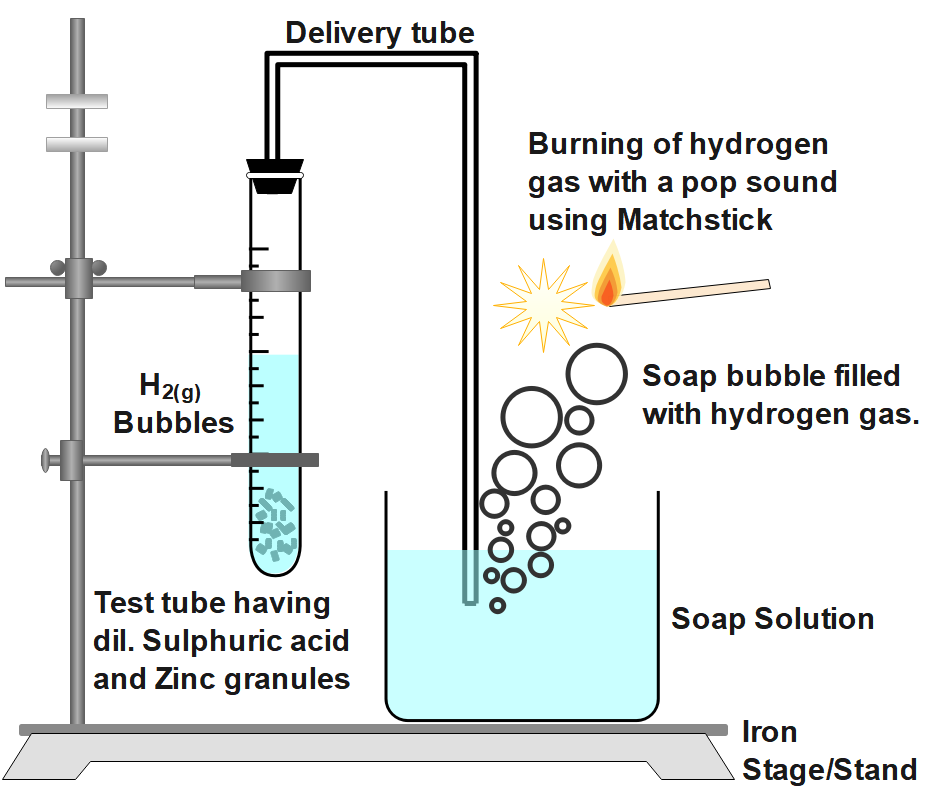
A.In place of zinc granules, the same amount of zinc dust is taken in the test tube.
B.Instead of dilute sulphuric acid, dilute hydrochloric acid is taken. Write the chemical reaction involved.
C.In place of zinc, copper turnings are taken.

Answer
498k+ views
Hint: We know that the metal (zinc granules) reacts with sulphuric acid to form metal sulphate and hydrogen gas. A metal reacts with hydrochloric acid to form metal chloride and hydrogen gas. As referred to in the above diagram, not many reactions are normally quicker than others while a few reactions are very slow. The rate of reaction is for the most part slower in fluids when contrasted with gases and slower in solids when contrasted with fluids.
Complete answer:
As we know that the protons accept electrons to form hydrogen gas. Thus, sulphuric acid or hydrochloric acid is reduced during the reaction. Gain of electrons is reduction. Metal is oxidized to metal ions in the salt as metal loses electrons to form metal cations. The loss of electrons is oxidation. Thus, above reactions represent redox reactions in which sulphuric acid or hydrochloric acid acts as oxidizing agent and metal acts as reducing agent.
If within the place of metal granules, an identical quantity of metal dirt is taken within the tubing then the speed of the reaction can increase and therefore the reaction can occur a lot of quickly. This can be because the extent per unit volume of powder is quite that of the metal granules and therefore the powder can give a lot of extent for the reaction to require place.
A.Instead of zinc granules if we use the same amount of zinc dust in the test tube there will be a certain amount of rising in rate of reaction. This raise in rate of reaction will lead into faster reaction i.e., the reaction will be quicker.
B.Likewise, instead of dil. Sulphuric acid if we use dil. Hydrochloric acid there will be no changes. Since both are acids which are dilute by nature and still will remain strong acids.
C.Now, if we use copper instead of zinc, the reaction will be the same since both are metals which will lead to formation of hydrogen gas. For that the reaction can be given as:
For Zinc: $Zn+H_{2}^{{}}S{{O}_{4}}\to ZnS{{O}_{4}}+{{H}_{2}}\uparrow $
For Copper: $Cu+H_{2}^{{}}S{{O}_{4}}\to CuS{{O}_{4}}+{{H}_{2}}\uparrow $
Note:
Remember that the higher temperature also increases the rate of the reaction. The reaction rate is highly affected by the solvent properties and ionic strength. Also, the rate of reaction is also affected by the surface area of the reactants.
Complete answer:
As we know that the protons accept electrons to form hydrogen gas. Thus, sulphuric acid or hydrochloric acid is reduced during the reaction. Gain of electrons is reduction. Metal is oxidized to metal ions in the salt as metal loses electrons to form metal cations. The loss of electrons is oxidation. Thus, above reactions represent redox reactions in which sulphuric acid or hydrochloric acid acts as oxidizing agent and metal acts as reducing agent.
If within the place of metal granules, an identical quantity of metal dirt is taken within the tubing then the speed of the reaction can increase and therefore the reaction can occur a lot of quickly. This can be because the extent per unit volume of powder is quite that of the metal granules and therefore the powder can give a lot of extent for the reaction to require place.
A.Instead of zinc granules if we use the same amount of zinc dust in the test tube there will be a certain amount of rising in rate of reaction. This raise in rate of reaction will lead into faster reaction i.e., the reaction will be quicker.
B.Likewise, instead of dil. Sulphuric acid if we use dil. Hydrochloric acid there will be no changes. Since both are acids which are dilute by nature and still will remain strong acids.
C.Now, if we use copper instead of zinc, the reaction will be the same since both are metals which will lead to formation of hydrogen gas. For that the reaction can be given as:
For Zinc: $Zn+H_{2}^{{}}S{{O}_{4}}\to ZnS{{O}_{4}}+{{H}_{2}}\uparrow $
For Copper: $Cu+H_{2}^{{}}S{{O}_{4}}\to CuS{{O}_{4}}+{{H}_{2}}\uparrow $
Note:
Remember that the higher temperature also increases the rate of the reaction. The reaction rate is highly affected by the solvent properties and ionic strength. Also, the rate of reaction is also affected by the surface area of the reactants.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




