
In the fractionating column of fractional distillation, as the column gets higher:
A) Temperature becomes lower
B) Temperature becomes higher
C) Minimum absorption is carried out
D) Risks of sublimation exist
Answer
583.2k+ views
Hint: The fractional distillation is the separation of liquid mixtures which have a small difference in their volatility. The fractionating column allows the separation by the continuous condensation and vaporization of the mixture. The hot vapour passes through the column and exchanges its heat energy with the walls. Such that, the column experiences the temperature gradient between the lower and upper end of the column.
Complete step by step answer:
The fractional distillation is used for the separation of miscible liquids. The fractional distillation is a process of continuous distillation and condensation of the components of the mixture. As the name suggests, the components are separated as the fraction. The mixture is heated such that the components start to vaporize at its boiling point and the fraction is separated.
When the mixture of miscible liquid is heated, the component which has the lower boiling point starts to boil first and separated first followed by the other components which have a higher boiling point.
The general apparatus is as shown below,
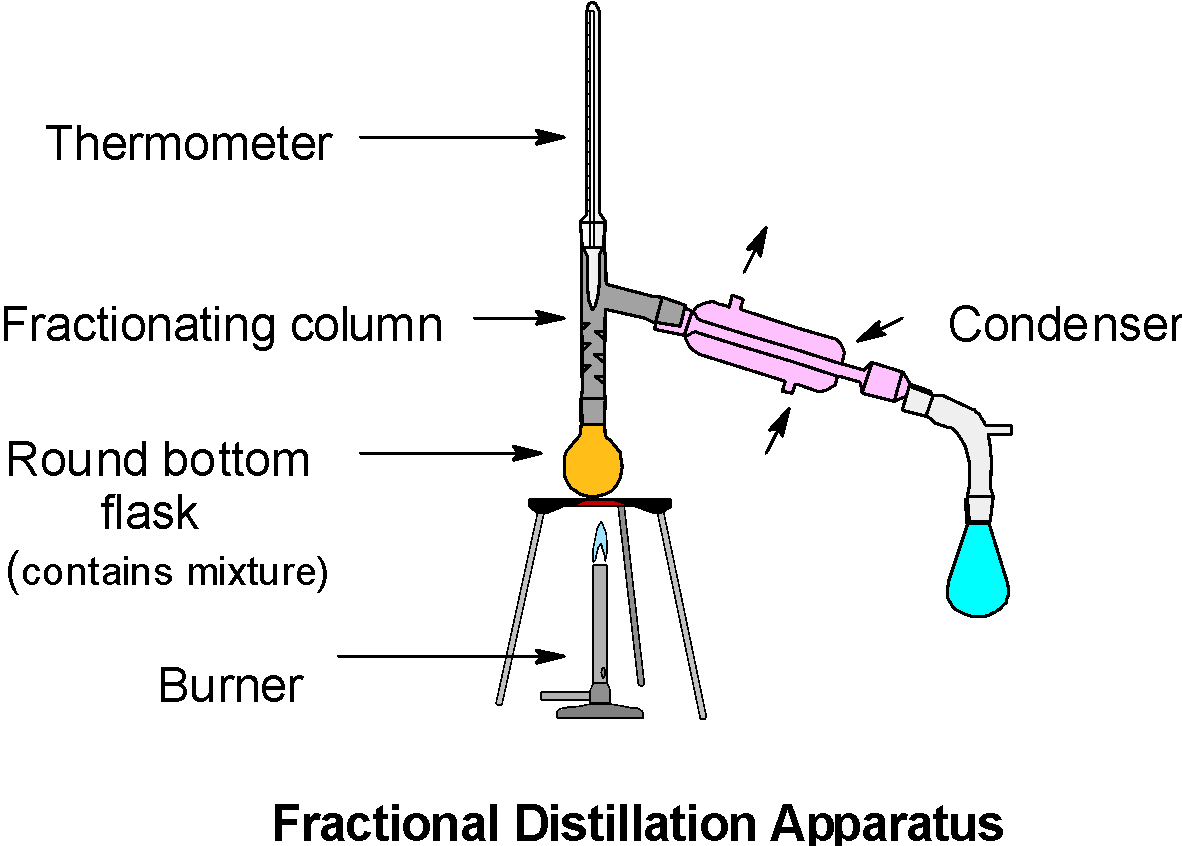
The fractional distillation apparatus consists of a fractionating column. It is a piece of glassware and used to separate the vaporized mixture. The fractional distillation is applicable for the mixture of liquids which have close volatility or close boiling point. The fractionating column allows the vapours of the mixture to cool, condense and evaporate again by Raoult’s law. The fractionating column allows the continuous condensation and vaporization of the mixture and enriches the separation of a component.
Now, consider a separation of a mixture by the fractional distillation. During the separation process, the hot vapour of the mixture passes through the column. As the hot vapour passes through the column, it transfers its heat energy to the walls of the column and condenses. This transfer of energy heats up the column. Thus, because of this transfer of energy, more vapours enter into the column than the vapours which leave the column.
Therefore, the lower end of the column has a higher temperature than that of the upper end (exit) of the fractionating column.
So, in the fractionating column of fractional distillation, as the column gets higher the temperature becomes lower.
So, the correct answer is “Option A”.
Note: Note that, if the boiling point of liquids is close, then the fractionating column should be high, it allows the better separation of the temperature. The fractionating column is filled with the glass bead. This increases the surface area and allows the more condensation - vaporization cycles and improves separation. There are various fractionating columns, such as the Vigreux column or packed column (filled with beads provides the large surface area).
Complete step by step answer:
The fractional distillation is used for the separation of miscible liquids. The fractional distillation is a process of continuous distillation and condensation of the components of the mixture. As the name suggests, the components are separated as the fraction. The mixture is heated such that the components start to vaporize at its boiling point and the fraction is separated.
When the mixture of miscible liquid is heated, the component which has the lower boiling point starts to boil first and separated first followed by the other components which have a higher boiling point.
The general apparatus is as shown below,

The fractional distillation apparatus consists of a fractionating column. It is a piece of glassware and used to separate the vaporized mixture. The fractional distillation is applicable for the mixture of liquids which have close volatility or close boiling point. The fractionating column allows the vapours of the mixture to cool, condense and evaporate again by Raoult’s law. The fractionating column allows the continuous condensation and vaporization of the mixture and enriches the separation of a component.
Now, consider a separation of a mixture by the fractional distillation. During the separation process, the hot vapour of the mixture passes through the column. As the hot vapour passes through the column, it transfers its heat energy to the walls of the column and condenses. This transfer of energy heats up the column. Thus, because of this transfer of energy, more vapours enter into the column than the vapours which leave the column.
Therefore, the lower end of the column has a higher temperature than that of the upper end (exit) of the fractionating column.
So, in the fractionating column of fractional distillation, as the column gets higher the temperature becomes lower.
So, the correct answer is “Option A”.
Note: Note that, if the boiling point of liquids is close, then the fractionating column should be high, it allows the better separation of the temperature. The fractionating column is filled with the glass bead. This increases the surface area and allows the more condensation - vaporization cycles and improves separation. There are various fractionating columns, such as the Vigreux column or packed column (filled with beads provides the large surface area).
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Difference Between Plant Cell and Animal Cell

Fill the blanks with the suitable prepositions 1 The class 9 english CBSE

Who is eligible for RTE class 9 social science CBSE

Which places in India experience sunrise first and class 9 social science CBSE

What is pollution? How many types of pollution? Define it

Name 10 Living and Non living things class 9 biology CBSE




