
In the manufacture of ammonia by Haber process high pressure is maintained because:
(A) all reactants and products are gases.
(B) reaction is exothermic.
(C) the moles of the product are less than the moles of reactants.
(D) reaction is reversible reaction.
Answer
581.1k+ views
Hint: Use Le chatelier's principle of chemical equilibrium which states that the changes in the temperature, pressure, volume or the concentration of a system results in a predictable and opposing changes in the system in order to achieve a new equilibrium state.
Complete answer:
In Haber’s process Ammonia is manufactured by nitrogen and hydrogen gas. The reaction is as follows:
$N_2(g) + 3H_2(g) \leftrightarrows 2NH_3(g)$
1 mole of nitrogen has reacted with 3 moles of Hydrogen gas and formed two moles of ammonia.
Maximum yield of ammonia depends on certain factors like pressure, temperature, concept reaction and catalyst.
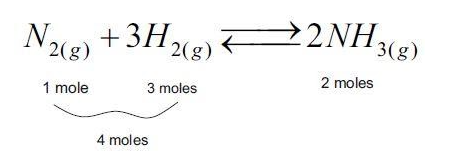
In reaction is in equilibrium state. It means reaction occurs in both directions.
If we want more ammonia, then high pressure should be applied.
When total moles of reactants are less than total moles of product then at high pressure equilibrium shift in forward direction.
Here we see that 4 moles of reactant produce 2 moles of product, therefore high pressure is applied to produce more ammonia.
At low pressure equilibrium shifts in backward direction and more ${N_2}$ and ${H_2}$ will be formed.
Therefore, from the above explanation the correct option is the moles of the product are less than the moles of reactants.
Thus the correct option is C.
Note: The more yield of ammonia depends on other factors too. They are,
(1) Concentration: If concentration of moles of reactants is more than moles of product then a equilibrium shifts to forward direction on increasing concentration of ${N_2}$ and ${H_2}$ and yield more ammonia.
(2) Temperature: Maximum yield of ammonia is obtained at optimum temperature of 700K. If the temperature is increased endothermic reaction is favoured and if the temperature is decreased exothermic reaction is favoured.
Complete answer:
In Haber’s process Ammonia is manufactured by nitrogen and hydrogen gas. The reaction is as follows:
$N_2(g) + 3H_2(g) \leftrightarrows 2NH_3(g)$
1 mole of nitrogen has reacted with 3 moles of Hydrogen gas and formed two moles of ammonia.
Maximum yield of ammonia depends on certain factors like pressure, temperature, concept reaction and catalyst.

In reaction is in equilibrium state. It means reaction occurs in both directions.
If we want more ammonia, then high pressure should be applied.
When total moles of reactants are less than total moles of product then at high pressure equilibrium shift in forward direction.
Here we see that 4 moles of reactant produce 2 moles of product, therefore high pressure is applied to produce more ammonia.
At low pressure equilibrium shifts in backward direction and more ${N_2}$ and ${H_2}$ will be formed.
Therefore, from the above explanation the correct option is the moles of the product are less than the moles of reactants.
Thus the correct option is C.
Note: The more yield of ammonia depends on other factors too. They are,
(1) Concentration: If concentration of moles of reactants is more than moles of product then a equilibrium shifts to forward direction on increasing concentration of ${N_2}$ and ${H_2}$ and yield more ammonia.
(2) Temperature: Maximum yield of ammonia is obtained at optimum temperature of 700K. If the temperature is increased endothermic reaction is favoured and if the temperature is decreased exothermic reaction is favoured.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




