
In the phase diagram shown, the point Q corresponds to the triple point of water the region \[{\text{I}}\], \[{\text{II}}\] and \[{\text{III}}\] respectively corresponds to phases.

a. Liquid, solid, vapor
b. Solid, liquid, vapor
c. Liquid, vapor, solid
d. Solid, vapor, liquid.

Answer
568.8k+ views
Hint: A substance can exist in all three phases- solids, liquid, and vapor under suitable conditions of temperature and pressure. With help of a phase diagram (P-T graph), the behavior of matter in different phases can be determined.
Complete step by step answer:
A Triple point is a point in the phase diagram representing a particular pressure and temperature at which the solid, liquid, and vapor can co-exist.
The solid (ice) and liquid (water) phases are in equilibrium at a temperature called the melting point or fusion point. Similarly, the temperature at which the liquid phase (water) and vapor phase (steam) are in equilibrium is called the boiling point. Similarly, the temperatures at which the solid phase (ice) and vapour phase (steam) are in equilibrium are called sublimation point. All these temperatures change with pressure. So, a phase diagram is obtained as shown in the figure.
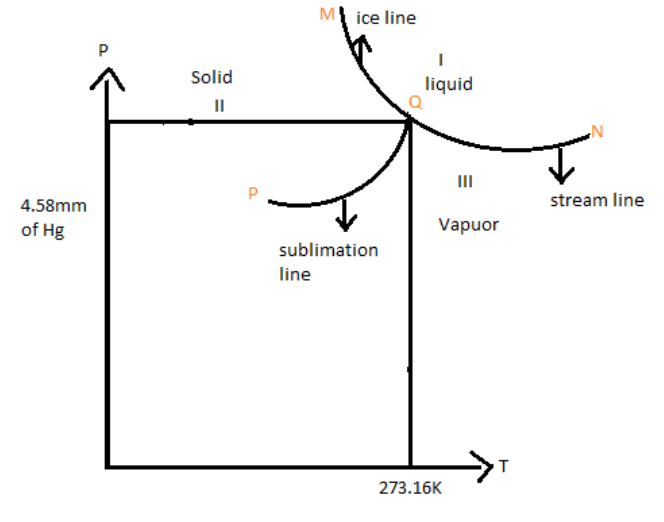
$\left( {\text{I}} \right)$ Steam line: in the diagram the curve QN, is called the steam line. Along the curve, the steam and water are in equilibrium. It shows the variation of the boiling point of water with pressure. Any slight deviation either in pressure or in temperature from any point on the curve we miss one of the phases bounded by the curve.
$\left( {{\text{II}}} \right)$ Ice line: the curve QM is called the ice line. Along the curve, the ice and water are in equilibrium. The curve shows the variation of the melting point of ice with pressure.
$\left( {{\text{III}}} \right)$ Sublimation line: the curve PQ is called the sublimation line. Along the curve, the ice and steam are in equilibrium. The curve shows the variation of sublimation point with pressure.
All the three lines QM, QN, and PQ meet at a single point called triple point of water.
At the corresponding temperature and pressure, the three phases of water co-exist.
Hence, the correct answer is option (A).
Note: If the temperature at triple point is kept constant pressure increases slightly then the phase is liquid. Sublimation is the process of direct change from a solid-state to the vapor state. The temperature at which a liquid converts into vapor is called the boiling point.
Complete step by step answer:
A Triple point is a point in the phase diagram representing a particular pressure and temperature at which the solid, liquid, and vapor can co-exist.
The solid (ice) and liquid (water) phases are in equilibrium at a temperature called the melting point or fusion point. Similarly, the temperature at which the liquid phase (water) and vapor phase (steam) are in equilibrium is called the boiling point. Similarly, the temperatures at which the solid phase (ice) and vapour phase (steam) are in equilibrium are called sublimation point. All these temperatures change with pressure. So, a phase diagram is obtained as shown in the figure.

$\left( {\text{I}} \right)$ Steam line: in the diagram the curve QN, is called the steam line. Along the curve, the steam and water are in equilibrium. It shows the variation of the boiling point of water with pressure. Any slight deviation either in pressure or in temperature from any point on the curve we miss one of the phases bounded by the curve.
$\left( {{\text{II}}} \right)$ Ice line: the curve QM is called the ice line. Along the curve, the ice and water are in equilibrium. The curve shows the variation of the melting point of ice with pressure.
$\left( {{\text{III}}} \right)$ Sublimation line: the curve PQ is called the sublimation line. Along the curve, the ice and steam are in equilibrium. The curve shows the variation of sublimation point with pressure.
All the three lines QM, QN, and PQ meet at a single point called triple point of water.
At the corresponding temperature and pressure, the three phases of water co-exist.
Hence, the correct answer is option (A).
Note: If the temperature at triple point is kept constant pressure increases slightly then the phase is liquid. Sublimation is the process of direct change from a solid-state to the vapor state. The temperature at which a liquid converts into vapor is called the boiling point.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




