
In the polyatomic ion, $CrO_4^{2 - }$ , what is the charge on the chromium atom?
Answer
491.7k+ views
Hint: To solve this problem we’ll have to know about the basic normal valencies of common elements such as Oxygen. It will be helpful if you remember the Oxidation States (charge) or other common elements like carbon, nitrogen, Hydrogen, etc.
Complete answer:
The stability of an atom is gained by losing or gaining electrons. The charge that is generated by losing or gaining electrons is known as the oxidation state. The charge of the Chromium atom in the given compound $CrO_4^{2 - }$ can be found out by considering the Oxidation state of Cr to be unknown ‘x’.
The overall charge of the compound $CrO_4^{2 - }$ is found to be -2. The common oxidation state of Oxygen is -2. Since we have 4 oxygen atoms, the oxidation state will be 4 times -2. Consider the Oxidation state of Cr in $CrO_4^{2 - }$ to be ‘x’. The overall charge on the compound can be given as:
$x + 4 \times ( - 2) = - 2$
$x - 8 = - 2$
$x = - 2 + 8$
$x = + 6$
Therefore, the charge on Chromium atoms in $CrO_4^{2 - }$ is +6. This ion given to us is the Chromate ion. The molecular structure of this ion is given below:
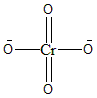
Note:
The compound given to us is chromate ion. The molecular weight of Chromate is 194 g/mol. Chromate ion is found to be more dominant in basic solution, but the dichromate ion is more dominant in acidic solutions.
In a compound, there is loss of gain or electrons happening. Once an atom loses/gains and electrons it becomes an ion. An ion can accept electrons only till its outermost shell is full. Beyond that no gain of electrons happens.
Complete answer:
The stability of an atom is gained by losing or gaining electrons. The charge that is generated by losing or gaining electrons is known as the oxidation state. The charge of the Chromium atom in the given compound $CrO_4^{2 - }$ can be found out by considering the Oxidation state of Cr to be unknown ‘x’.
The overall charge of the compound $CrO_4^{2 - }$ is found to be -2. The common oxidation state of Oxygen is -2. Since we have 4 oxygen atoms, the oxidation state will be 4 times -2. Consider the Oxidation state of Cr in $CrO_4^{2 - }$ to be ‘x’. The overall charge on the compound can be given as:
$x + 4 \times ( - 2) = - 2$
$x - 8 = - 2$
$x = - 2 + 8$
$x = + 6$
Therefore, the charge on Chromium atoms in $CrO_4^{2 - }$ is +6. This ion given to us is the Chromate ion. The molecular structure of this ion is given below:

Note:
The compound given to us is chromate ion. The molecular weight of Chromate is 194 g/mol. Chromate ion is found to be more dominant in basic solution, but the dichromate ion is more dominant in acidic solutions.
In a compound, there is loss of gain or electrons happening. Once an atom loses/gains and electrons it becomes an ion. An ion can accept electrons only till its outermost shell is full. Beyond that no gain of electrons happens.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




