
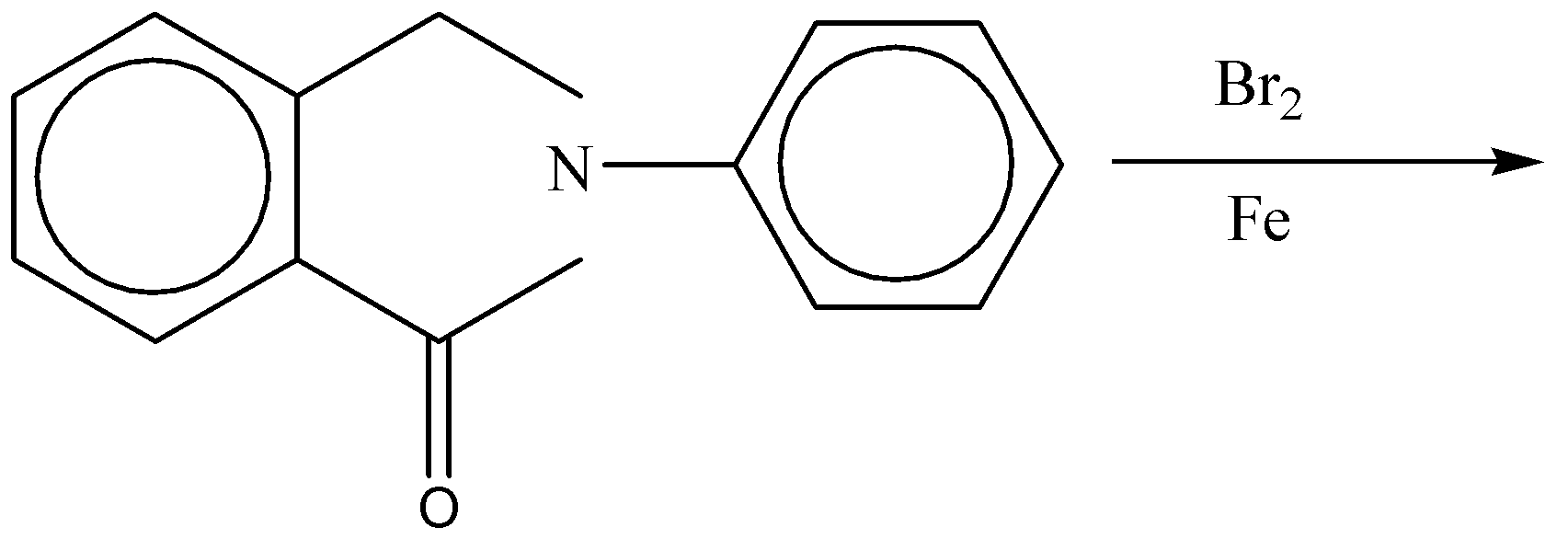
In the reaction the major product formed is:

Answer
573.9k+ views
Hint: This is electrophilic aromatic substitution reaction in which hydrogen atom attached to the ring will be replaced with the bromine atom. The electrophilic substitution will take place at an electron rich position.
Complete step by step answer:
The electrophilic substitution takes place at electron rich position. When the benzene reacts with the bromine under some harsh conditions like liquid bromine, no solvent and the Lewis acid \[FeB{r_3}\] as a catalyst, hen a reaction occurs in which one bromine is substituted for a ring hydrogen.
The above reaction is an example of electrophilic aromatic substitution reaction in which hydrogen atom attached to the ring is replaced with bromine atom. Only one benzene ring will undergo the substitution while the other benzene ring will not be substituted. The benzene ring in which the\[-NHCO\] group is present will be substituted and the bromine atom will occupy the para position to \[-NHCO\] group. Since the right-hand side ring is activated means electron rich as nitrogen is directly attached to the ring.
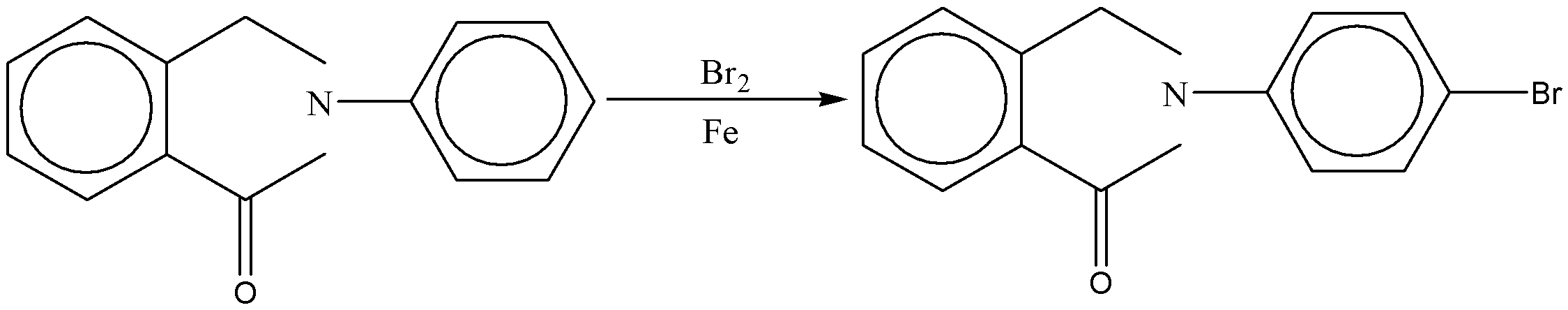
Hence, the major product of the reaction is shown below.
Note: The organic reactions where an electrophile replaces an atom that is attached to an aromatic ring is known as electrophilic substitution reaction. In these reactions, the hydrogen atom belonging to the benzene ring (aromatic compound) is replaced with an electrophile (electron poor species). This reaction of benzene with halogens \[\left( {B{r_{2,}}C{l_2}} \right)\] is different from the reaction of alkenes with halogens in two ways such as the product obtained and alkenes reacts spontaneously with \[B{r_{2,}}\] or \[C{l_2}\] even in the dilute solution to give addition products.
Complete step by step answer:
The electrophilic substitution takes place at electron rich position. When the benzene reacts with the bromine under some harsh conditions like liquid bromine, no solvent and the Lewis acid \[FeB{r_3}\] as a catalyst, hen a reaction occurs in which one bromine is substituted for a ring hydrogen.
The above reaction is an example of electrophilic aromatic substitution reaction in which hydrogen atom attached to the ring is replaced with bromine atom. Only one benzene ring will undergo the substitution while the other benzene ring will not be substituted. The benzene ring in which the\[-NHCO\] group is present will be substituted and the bromine atom will occupy the para position to \[-NHCO\] group. Since the right-hand side ring is activated means electron rich as nitrogen is directly attached to the ring.
Hence, the major product of the reaction is shown below.

Note: The organic reactions where an electrophile replaces an atom that is attached to an aromatic ring is known as electrophilic substitution reaction. In these reactions, the hydrogen atom belonging to the benzene ring (aromatic compound) is replaced with an electrophile (electron poor species). This reaction of benzene with halogens \[\left( {B{r_{2,}}C{l_2}} \right)\] is different from the reaction of alkenes with halogens in two ways such as the product obtained and alkenes reacts spontaneously with \[B{r_{2,}}\] or \[C{l_2}\] even in the dilute solution to give addition products.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




