
Answer
460.5k+ views
Hint: Generally, tetrahedral voids are two times octahedral voids in cubic close packed structures.
Complete step by step solution:
Let us first understand about the packed structures and voids present within them.
Cubic close packed structures-
The cubic close packed structure consists of four layers of atoms per unit cell. There are two types of arrangement of atoms in the structure i.e. Simple cubic arrangement and Hexagonal cubic arrangement.
Simple cubic arrangement has the same layer of atoms arranged layer by layer one above the other in a definite manner.
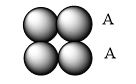
Hexagonal cubic arrangement has one layer in the depression of another layer, repeating over one another.
There are two types of gaps (commonly known as voids) within them;
Tetrahedral void-
These are mostly found in CCP and are triangular in shape with coordination number of 4.
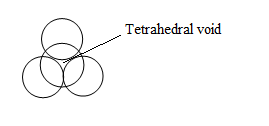
Tetrahedral voids are two times the number of atoms in the unit cell.
Octahedral void-
These are mostly found in HCP and are octahedral in shape with coordination number of 6.
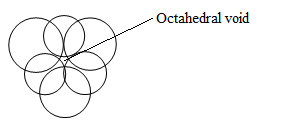
Octahedral voids are equal to the number of atoms in the unit cell.
Thus,
Tetrahedral voids are two times the octahedral voids.
Spinel group-
These are the group of minerals with general formulation $A{{B}_{2}}{{X}_{4}}$.
where,
X is anion
A and B are cations.
Illustration,
Oxides form close packings. Thus, it is an anion.
Whereas, ${{A}^{2+}}$ and ${{B}^{3+}}$ are cations.
Given that,
${{\dfrac{1}{8}}^{th}}$of tetrahedral voids are occupied by ${{A}^{2+}}$cation;
Thus, ${{A}^{2+}}$ ions will be ${{\dfrac{1}{4}}^{th}}$of oxide ions.
And half of octahedral voids are occupied by ${{B}^{3+}}$cations;
Thus, ${{B}^{3+}}$ ions will be $\dfrac{1}{2}$ of oxide ions.
Hence, A:B:O = $\dfrac{1}{4}$: $\dfrac{1}{2}$: 1 = 1:2:4.
Therefore, the formula is $A{{B}_{2}}{{O}_{4}}$.
Option (B) is correct.
Note: In accordance with the hint given, we can ignore option (A) and (D) from the start. Only concentrate on the remaining two options.
Complete step by step solution:
Let us first understand about the packed structures and voids present within them.
Cubic close packed structures-
The cubic close packed structure consists of four layers of atoms per unit cell. There are two types of arrangement of atoms in the structure i.e. Simple cubic arrangement and Hexagonal cubic arrangement.
Simple cubic arrangement has the same layer of atoms arranged layer by layer one above the other in a definite manner.

Hexagonal cubic arrangement has one layer in the depression of another layer, repeating over one another.

There are two types of gaps (commonly known as voids) within them;
Tetrahedral void-
These are mostly found in CCP and are triangular in shape with coordination number of 4.

Tetrahedral voids are two times the number of atoms in the unit cell.
Octahedral void-
These are mostly found in HCP and are octahedral in shape with coordination number of 6.

Octahedral voids are equal to the number of atoms in the unit cell.
Thus,
Tetrahedral voids are two times the octahedral voids.
Spinel group-
These are the group of minerals with general formulation $A{{B}_{2}}{{X}_{4}}$.
where,
X is anion
A and B are cations.
Illustration,
Oxides form close packings. Thus, it is an anion.
Whereas, ${{A}^{2+}}$ and ${{B}^{3+}}$ are cations.
Given that,
${{\dfrac{1}{8}}^{th}}$of tetrahedral voids are occupied by ${{A}^{2+}}$cation;
Thus, ${{A}^{2+}}$ ions will be ${{\dfrac{1}{4}}^{th}}$of oxide ions.
And half of octahedral voids are occupied by ${{B}^{3+}}$cations;
Thus, ${{B}^{3+}}$ ions will be $\dfrac{1}{2}$ of oxide ions.
Hence, A:B:O = $\dfrac{1}{4}$: $\dfrac{1}{2}$: 1 = 1:2:4.
Therefore, the formula is $A{{B}_{2}}{{O}_{4}}$.
Option (B) is correct.
Note: In accordance with the hint given, we can ignore option (A) and (D) from the start. Only concentrate on the remaining two options.
Recently Updated Pages
Fill in the blanks with suitable prepositions Break class 10 english CBSE

Fill in the blanks with suitable articles Tribune is class 10 english CBSE

Rearrange the following words and phrases to form a class 10 english CBSE

Select the opposite of the given word Permit aGive class 10 english CBSE

Fill in the blank with the most appropriate option class 10 english CBSE

Some places have oneline notices Which option is a class 10 english CBSE

Trending doubts
Fill the blanks with the suitable prepositions 1 The class 9 english CBSE

How do you graph the function fx 4x class 9 maths CBSE

When was Karauli Praja Mandal established 11934 21936 class 10 social science CBSE

Which are the Top 10 Largest Countries of the World?

What is the definite integral of zero a constant b class 12 maths CBSE

Why is steel more elastic than rubber class 11 physics CBSE

Distinguish between the following Ferrous and nonferrous class 9 social science CBSE

The Equation xxx + 2 is Satisfied when x is Equal to Class 10 Maths

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE




