
In which case geometrical isomerism is not exhibited?
A. Hyponitrous acid
B. 2 -butene
C. 1-butene
D. 2 -pentene
Answer
578.4k+ views
Hint: The compounds having the same chemical formula but a different arrangement of atoms is known as isomers. Geometrical isomerism is shown by the compound having unsaturation in which rotation around the unsaturated bond is restricted.
Complete step by step answer:
Geometrical isomerism is shown by the compounds that have a different arrangement of atoms around the unsaturated bond. The isomers are known as cis and trans.
Cis isomer is the isomer that has the same priority group on the same side of the unsaturated bond.
Trans isomer is the isomer that has the same priority groups on different sides of the unsaturated bond.
The isomerism exhibited by hyponitrous acid is as follows:
The chemical formula of hyponitrous acid is ${\text{OH}} - {\text{N}} = {\text{N}} - {\text{OH}}$ .
Both nitrogen atoms have one lone pair of electrons and one hydroxyl group.
The one structure that has both lone pairs on the same side or both hydroxyl groups on the same side of the double bond is the cis isomer.
The one structure that has both lone pairs on a different side or both hydroxyl groups on a different side of the double bond is the trans isomer
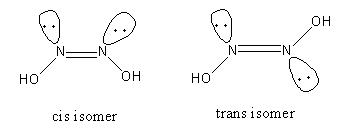
So, hyponitrous acid shows the geometrical isomerism so, option (A) is incorrect.
The isomerism exhibited by 2 -butene is as follows:
The chemical formula of $2$ -butene is ${\text{C}}{{\text{H}}_3} - {\text{CH}} = {\text{CH}} - {\text{C}}{{\text{H}}_3}$ .
Both double-bonded carbon atoms have one hydrogen atom and one methyl group.
The one structure that has both hydrogens on the same side or both methyl groups on the same side of the double bond is the cis isomer.
The one structure that has both hydrogens on a different side or both methyl groups on a different side of the double bond is the trans isomer.
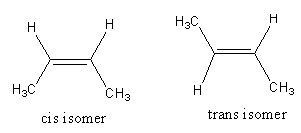
So, 2 -butene shows the geometrical isomerism so, option (B) is incorrect.
The isomerism exhibited by 1 -butene is as follows:
The chemical formula of 1 -butene is ${\text{C}}{{\text{H}}_3} - {\text{C}}{{\text{H}}_2} - {\text{CH = }}\,{\text{C}}{{\text{H}}_2}$ .
One carbon atom of double-bonded has two hydrogens and another carbon atom has one hydrogen atom and one ethyl group so, 1 -butane cannot show geometrical isomerism.
So, 1 -butene does not shows the geometrical isomerism so, option (C) is correct.
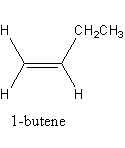
The isomerism exhibited by 2 -pentene is as follows:
The chemical formula of 2 -pentene is \[{\text{C}}{{\text{H}}_3} - {\text{CH}} = {\text{CH}} - {\text{C}}{{\text{H}}_2} - {\text{C}}{{\text{H}}_3}\] .
Both double-bonded carbon atoms have one hydrogen atom and one methyl group.
The one structure that has both hydrogens on the same side of the double bond is the cis isomer.
The one structure that has both hydrogens on a different side of the double bond is the trans isomer.
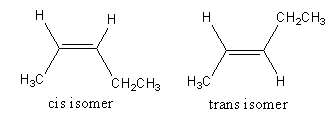
So, 2 - pentene shows the geometrical isomerism so, option (D) is incorrect.
Therefore, option (C) 1-butene, is correct.
Note: To show the geometrical isomerism each atom of the unsaturated bond should have two different groups. In 1 -butene, one carbon of double bond has two hydrogen atoms which will give the same structure concerning the hydrogen and methyl group of another double-bonded carbon atom.
Complete step by step answer:
Geometrical isomerism is shown by the compounds that have a different arrangement of atoms around the unsaturated bond. The isomers are known as cis and trans.
Cis isomer is the isomer that has the same priority group on the same side of the unsaturated bond.
Trans isomer is the isomer that has the same priority groups on different sides of the unsaturated bond.
The isomerism exhibited by hyponitrous acid is as follows:
The chemical formula of hyponitrous acid is ${\text{OH}} - {\text{N}} = {\text{N}} - {\text{OH}}$ .
Both nitrogen atoms have one lone pair of electrons and one hydroxyl group.
The one structure that has both lone pairs on the same side or both hydroxyl groups on the same side of the double bond is the cis isomer.
The one structure that has both lone pairs on a different side or both hydroxyl groups on a different side of the double bond is the trans isomer

So, hyponitrous acid shows the geometrical isomerism so, option (A) is incorrect.
The isomerism exhibited by 2 -butene is as follows:
The chemical formula of $2$ -butene is ${\text{C}}{{\text{H}}_3} - {\text{CH}} = {\text{CH}} - {\text{C}}{{\text{H}}_3}$ .
Both double-bonded carbon atoms have one hydrogen atom and one methyl group.
The one structure that has both hydrogens on the same side or both methyl groups on the same side of the double bond is the cis isomer.
The one structure that has both hydrogens on a different side or both methyl groups on a different side of the double bond is the trans isomer.

So, 2 -butene shows the geometrical isomerism so, option (B) is incorrect.
The isomerism exhibited by 1 -butene is as follows:
The chemical formula of 1 -butene is ${\text{C}}{{\text{H}}_3} - {\text{C}}{{\text{H}}_2} - {\text{CH = }}\,{\text{C}}{{\text{H}}_2}$ .
One carbon atom of double-bonded has two hydrogens and another carbon atom has one hydrogen atom and one ethyl group so, 1 -butane cannot show geometrical isomerism.
So, 1 -butene does not shows the geometrical isomerism so, option (C) is correct.

The isomerism exhibited by 2 -pentene is as follows:
The chemical formula of 2 -pentene is \[{\text{C}}{{\text{H}}_3} - {\text{CH}} = {\text{CH}} - {\text{C}}{{\text{H}}_2} - {\text{C}}{{\text{H}}_3}\] .
Both double-bonded carbon atoms have one hydrogen atom and one methyl group.
The one structure that has both hydrogens on the same side of the double bond is the cis isomer.
The one structure that has both hydrogens on a different side of the double bond is the trans isomer.

So, 2 - pentene shows the geometrical isomerism so, option (D) is incorrect.
Therefore, option (C) 1-butene, is correct.
Note: To show the geometrical isomerism each atom of the unsaturated bond should have two different groups. In 1 -butene, one carbon of double bond has two hydrogen atoms which will give the same structure concerning the hydrogen and methyl group of another double-bonded carbon atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




