
In which of the following compounds, cross conjugation are present?
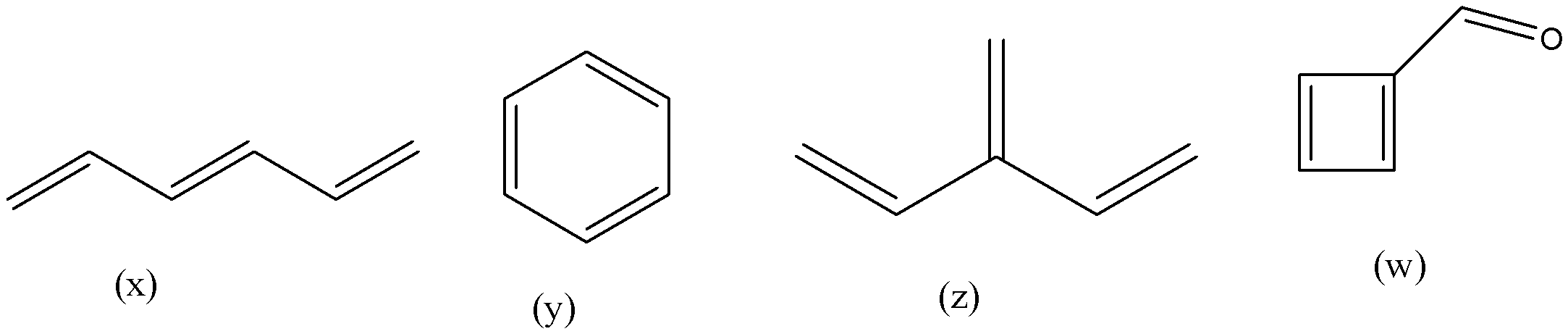
(a)- x, y
(b)- y, z, w
(c)- x, y, z, w
(d)- Only z

Answer
569.1k+ views
Hint: Cross-conjugation is a type of conjugation in which there are three pi-bond systems and these are in conjugation. The conjugation is in such a way that the first pi-system is in conjugation with the second pi-system and the second pi-system is in conjugation with the third pi-system but the first and third pi-systems are not in conjugation with each other.
Complete Solution :
The conjugation system of pi-bonds in compounds means there are alternate single and double bonds in the compound. 1, 3-Butadiene is an example of a conjugated system. There are two bonds and one single bond in 1, 3-Butadiene. The formula is given:
$C{{H}_{2}}=CH-CH=C{{H}_{2}}$
- Cross-conjugation is a type of conjugation in which there are three pi-bond systems and these are in conjugation. The conjugation is in such a way that the first pi-system is in conjugation with the second pi-system and the second pi-system is in conjugation with the third pi-system but the first and third pi-systems are not in conjugation with each other.
So, from the given compounds in the question:
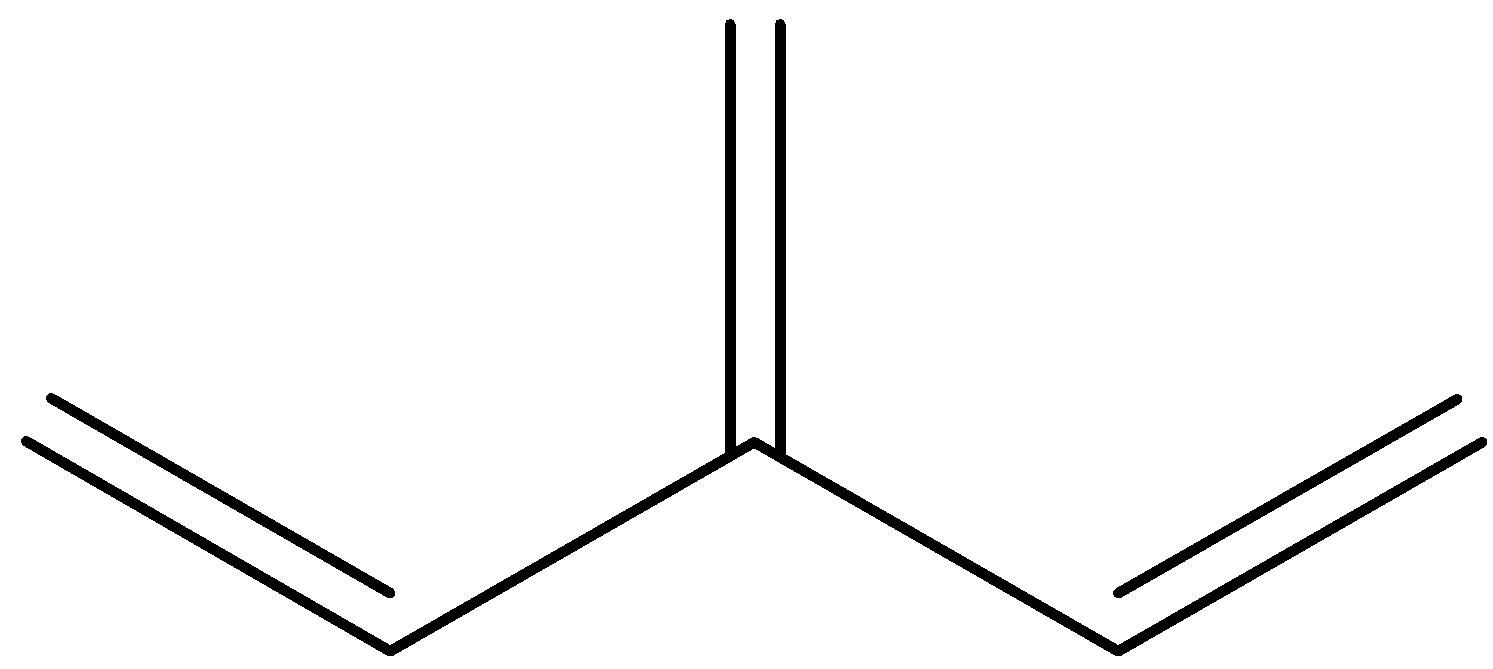
Is an example of a cross-conjugation system. Let us give numbers to all the pi-bond in the system:
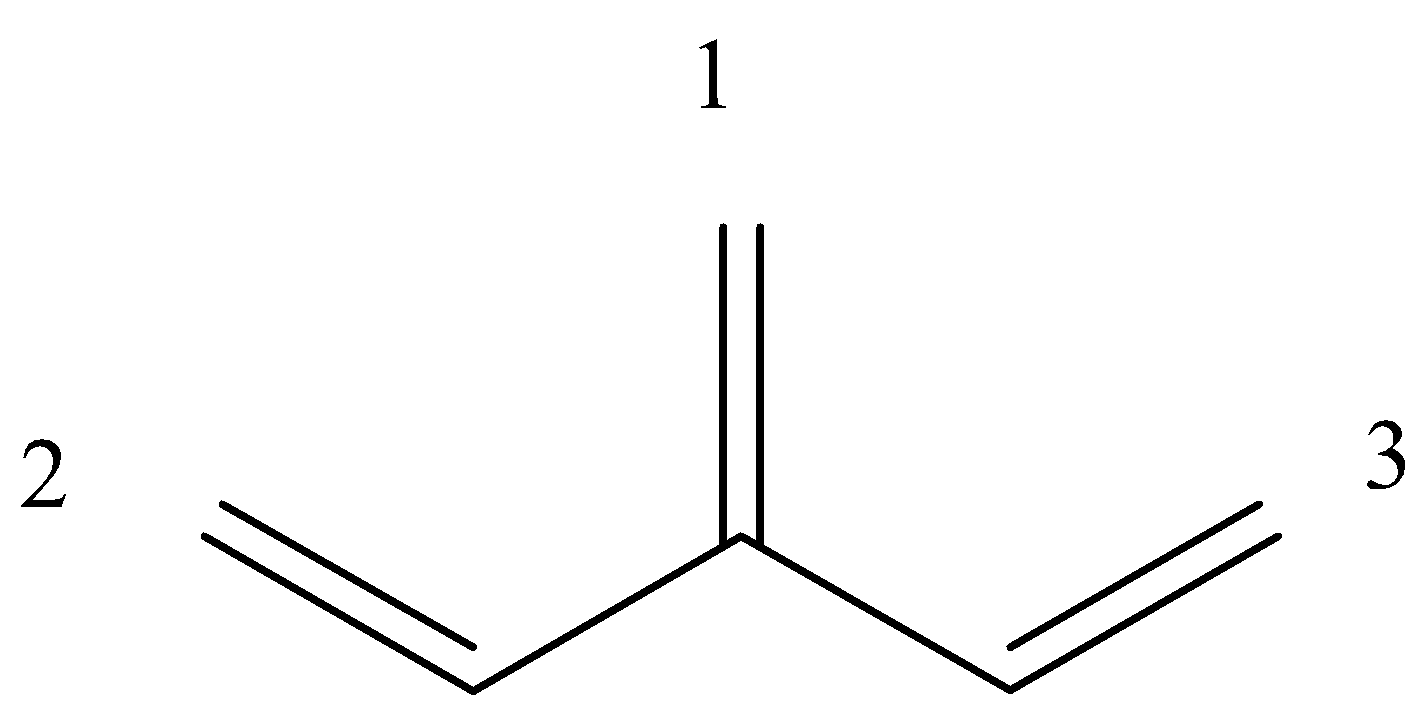
So, we can see that 1 and 2 are in conjugation, and 1 and 3 are in conjugation, but 2 and 3 are not in conjugation because there are 2 single bonds between them.
So, the correct answer is “Option D”.
Note: It must be noted that only two systems must be in the main chain and the third system must be in the side chain. Some other examples of cross-conjugation are benzophenone, divinyl ketones, fullerene, etc.
Complete Solution :
The conjugation system of pi-bonds in compounds means there are alternate single and double bonds in the compound. 1, 3-Butadiene is an example of a conjugated system. There are two bonds and one single bond in 1, 3-Butadiene. The formula is given:
$C{{H}_{2}}=CH-CH=C{{H}_{2}}$
- Cross-conjugation is a type of conjugation in which there are three pi-bond systems and these are in conjugation. The conjugation is in such a way that the first pi-system is in conjugation with the second pi-system and the second pi-system is in conjugation with the third pi-system but the first and third pi-systems are not in conjugation with each other.
So, from the given compounds in the question:

Is an example of a cross-conjugation system. Let us give numbers to all the pi-bond in the system:

So, we can see that 1 and 2 are in conjugation, and 1 and 3 are in conjugation, but 2 and 3 are not in conjugation because there are 2 single bonds between them.
So, the correct answer is “Option D”.
Note: It must be noted that only two systems must be in the main chain and the third system must be in the side chain. Some other examples of cross-conjugation are benzophenone, divinyl ketones, fullerene, etc.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




