
In which of the following is the oxidation number fractional?
(This question has multiple correct options)
(A) ${{B}_{4}}{{H}_{10}}$
(B) ${{B}_{2}}{{O}_{3}}$
(C) ${{C}_{5}}{{O}_{2}}$
(D) $K{{O}_{3}}$
Answer
565.2k+ views
Hint: Oxidation number of an atom is the total number of electrons that an atom either gains or losses in order to form a chemical bond with another atom. The oxidation number of an unknown atom in a molecule can be found if the oxidation numbers of the rest atoms are known.
Complete Solution :
So in the question four compounds are given and we have to find which of the compounds have fractional oxidation number. In the question itself it is given that there are multiple correct options for this question.
- We know that, the oxidation number or oxidation state of an atom describes the degree of oxidation (loss of electrons) or the degree of reduction (gain of electrons) in a chemical compound. Conceptually, the oxidation state, which may be positive, negative or zero, is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic, with no covalent component.
- In the given question, we will need to investigate each option individually:
(A)- ${{B}_{4}}{{H}_{10}}$
The ${{B}_{4}}{{H}_{10}}$ is a covalent compound which has four B-H-B bridges, no B-B-B triple bridge, one B-B bond and two terminal $B{{H}_{2}}$ groups. It is known as tetraborane. The structure of tetraborane is:
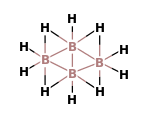
Now, we can calculate the oxidation number of B in this compound.
We know that there are four Boron atoms and ten oxide ions. Oxide ion possess a -2 charge hence we can find the oxidation state of B as,
$4x+10\left( -2 \right)=0$
The oxidation state of a compound is zero.
$4x+\left( -20 \right)=0$
$\Rightarrow$ $4x=20$ $x=\dfrac{20}{4}=5$
$\Rightarrow$ $x=\dfrac{20}{4}=+5$
The oxidation state of B is +5.
(B) ${{B}_{2}}{{O}_{3}}$
${{B}_{2}}{{O}_{3}}$ is a covalent compound which is a white, glassy solid. It is known as boron trioxide. The structure of boron trioxide is:
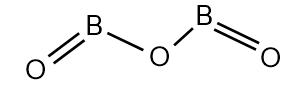
Let’s calculate the oxidation number of B in ${{B}_{2}}{{O}_{3}}$. There is two B atoms and three oxide ions in${{B}_{2}}{{O}_{3}}$
$\Rightarrow$ $2x+3\left( -2 \right)=0$
$\Rightarrow$ $2x+\left( -6 \right)=0$
$\Rightarrow$ $2x=6$
$\Rightarrow$ $x=\dfrac{6}{2}=+3$
Hence in${{B}_{2}}{{O}_{3}}$, B is having an oxidation state of +3.
(C) ${{C}_{5}}{{O}_{2}}$
The compound ${{C}_{5}}{{O}_{2}}$ is known as pentacarbon dioxide. It is stable at room temperature in solution. The structure of pentacarbon dioxide is:
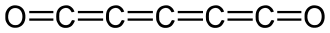
Let’s calculate the oxidation state of C in ${{C}_{5}}{{O}_{2}}$. There are five carbon atoms and two oxide ions.
Hence oxidation number is,
$5x+2\left( -2 \right)=0$
$\Rightarrow$ $5x+\left( -4 \right)=0$
$\Rightarrow$ $5x=+4$
$\Rightarrow$ $x=\dfrac{+4}{5}$
Here the C is having a fraction as an oxidation number. Hence this is one of the correct answers for the question.
(D) $K{{O}_{3}}$
$K{{O}_{3}}$ is an ionic compound. The potassium always has a +1 oxidation state. The oxidation state of oxygen can be found.
$\Rightarrow$ $1+3x=0$
$\Rightarrow$ $3x=-1$
$\Rightarrow$ $x=\dfrac{-1}{3}$
Here also the oxidation number obtained is a fractional number.
So, the correct answer is “Option C and D”.
Note: We should know the oxidation number of at least one atom then only we could calculate the oxidation state of the unknown, it is necessary to know the oxidation states of common atoms.
While calculating the oxidation states the sign should be always taken into consideration.
- Some students mix up valency and oxidation number, valency is a fixed number but oxidation number varies and in valency the sign does not have significance but it’s important in oxidation number.
Complete Solution :
So in the question four compounds are given and we have to find which of the compounds have fractional oxidation number. In the question itself it is given that there are multiple correct options for this question.
- We know that, the oxidation number or oxidation state of an atom describes the degree of oxidation (loss of electrons) or the degree of reduction (gain of electrons) in a chemical compound. Conceptually, the oxidation state, which may be positive, negative or zero, is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic, with no covalent component.
- In the given question, we will need to investigate each option individually:
(A)- ${{B}_{4}}{{H}_{10}}$
The ${{B}_{4}}{{H}_{10}}$ is a covalent compound which has four B-H-B bridges, no B-B-B triple bridge, one B-B bond and two terminal $B{{H}_{2}}$ groups. It is known as tetraborane. The structure of tetraborane is:

Now, we can calculate the oxidation number of B in this compound.
We know that there are four Boron atoms and ten oxide ions. Oxide ion possess a -2 charge hence we can find the oxidation state of B as,
$4x+10\left( -2 \right)=0$
The oxidation state of a compound is zero.
$4x+\left( -20 \right)=0$
$\Rightarrow$ $4x=20$ $x=\dfrac{20}{4}=5$
$\Rightarrow$ $x=\dfrac{20}{4}=+5$
The oxidation state of B is +5.
(B) ${{B}_{2}}{{O}_{3}}$
${{B}_{2}}{{O}_{3}}$ is a covalent compound which is a white, glassy solid. It is known as boron trioxide. The structure of boron trioxide is:

Let’s calculate the oxidation number of B in ${{B}_{2}}{{O}_{3}}$. There is two B atoms and three oxide ions in${{B}_{2}}{{O}_{3}}$
$\Rightarrow$ $2x+3\left( -2 \right)=0$
$\Rightarrow$ $2x+\left( -6 \right)=0$
$\Rightarrow$ $2x=6$
$\Rightarrow$ $x=\dfrac{6}{2}=+3$
Hence in${{B}_{2}}{{O}_{3}}$, B is having an oxidation state of +3.
(C) ${{C}_{5}}{{O}_{2}}$
The compound ${{C}_{5}}{{O}_{2}}$ is known as pentacarbon dioxide. It is stable at room temperature in solution. The structure of pentacarbon dioxide is:
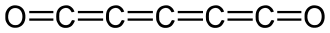
Let’s calculate the oxidation state of C in ${{C}_{5}}{{O}_{2}}$. There are five carbon atoms and two oxide ions.
Hence oxidation number is,
$5x+2\left( -2 \right)=0$
$\Rightarrow$ $5x+\left( -4 \right)=0$
$\Rightarrow$ $5x=+4$
$\Rightarrow$ $x=\dfrac{+4}{5}$
Here the C is having a fraction as an oxidation number. Hence this is one of the correct answers for the question.
(D) $K{{O}_{3}}$
$K{{O}_{3}}$ is an ionic compound. The potassium always has a +1 oxidation state. The oxidation state of oxygen can be found.
$\Rightarrow$ $1+3x=0$
$\Rightarrow$ $3x=-1$
$\Rightarrow$ $x=\dfrac{-1}{3}$
Here also the oxidation number obtained is a fractional number.
So, the correct answer is “Option C and D”.
Note: We should know the oxidation number of at least one atom then only we could calculate the oxidation state of the unknown, it is necessary to know the oxidation states of common atoms.
While calculating the oxidation states the sign should be always taken into consideration.
- Some students mix up valency and oxidation number, valency is a fixed number but oxidation number varies and in valency the sign does not have significance but it’s important in oxidation number.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




