
In which of the following molecules all atoms are not coplanar?
- A.
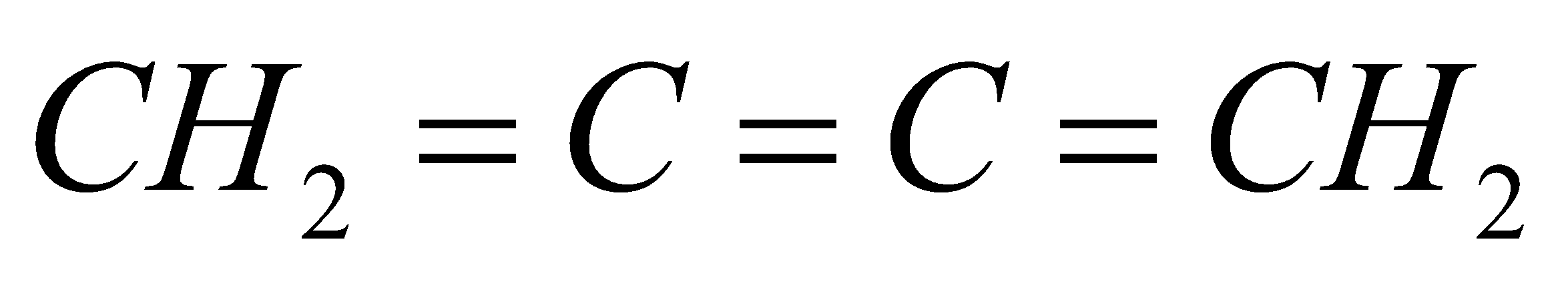
B.
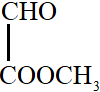
C.
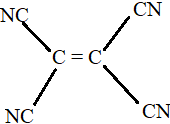
D.
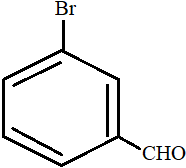
- A.
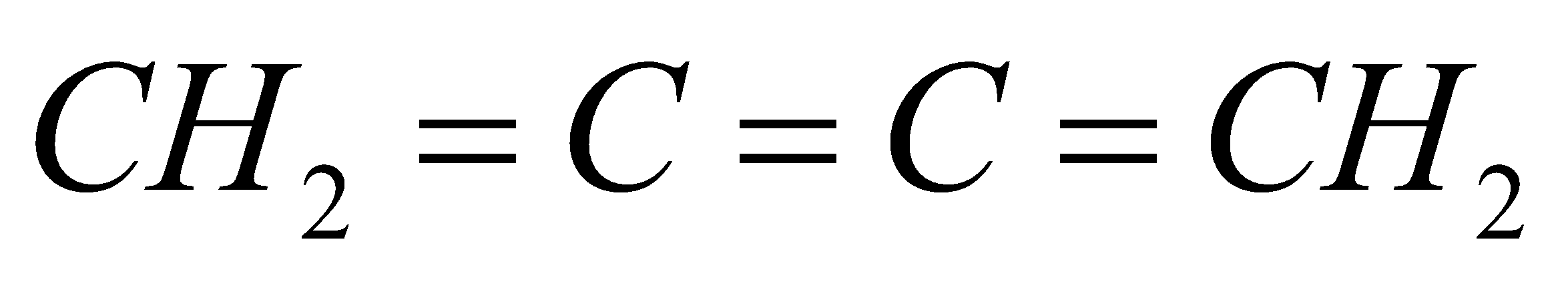
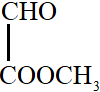


Answer
526.6k+ views
Hint- In order to deal with this question we will use the basic concept of organic chemistry which states that when a carbon atom is $s{p^2}$ or $sp$ hybridized, all the atoms attached to it are in a plane. In this question we will proceed further by understanding when carbon is said to be $sp/s{p^2}/s{p^3}$.
Complete answer:
$sp$ : The third possible arrangement for carbon is sp hybridization which occurs when carbon is bound to two other atoms (two double bonds or one single + one triple bond).
$s{p^2}$ : carbon is said to be $s{p^2}$ hybridized when it is DOUBLY BONDED with any 1 atom and SINGLY BONDED with any other 2 atoms.
$s{p^3}$ : The term “ $s{p^3}$ hybridization” refers to the mixing character of one 2s-orbitals and three 2p-orbitals to create four hybrid orbitals with similar characteristics. In order for an atom to be $s{p^3}$ hybridized, it must have an s orbital and three p orbitals
We know that when a carbon atom is $s{p^2}$ or $sp$ hybridized, all the atoms attached to it are in a plane.
All the carbon atoms in compounds A, C and D are $s{p^2}$ hybridized. Hence, in these molecules, all the atoms are coplanar.
In option B, one carbon atom is $s{p^3}$ hybridized. It has tetrahedral geometry. Hence, in this molecule, all atoms are not coplanar.
So, the correct answer is option B.
Note- Coplanar means atoms or groups of atoms that lie on the same plane. Like biphenyl, it has both benzene rings on the same plane. When all atoms of a compound are in the same plane are called coplanar compounds. Co planarity in organic compounds is seen in unsaturated molecules.
Complete answer:
$sp$ : The third possible arrangement for carbon is sp hybridization which occurs when carbon is bound to two other atoms (two double bonds or one single + one triple bond).
$s{p^2}$ : carbon is said to be $s{p^2}$ hybridized when it is DOUBLY BONDED with any 1 atom and SINGLY BONDED with any other 2 atoms.
$s{p^3}$ : The term “ $s{p^3}$ hybridization” refers to the mixing character of one 2s-orbitals and three 2p-orbitals to create four hybrid orbitals with similar characteristics. In order for an atom to be $s{p^3}$ hybridized, it must have an s orbital and three p orbitals
We know that when a carbon atom is $s{p^2}$ or $sp$ hybridized, all the atoms attached to it are in a plane.
All the carbon atoms in compounds A, C and D are $s{p^2}$ hybridized. Hence, in these molecules, all the atoms are coplanar.
In option B, one carbon atom is $s{p^3}$ hybridized. It has tetrahedral geometry. Hence, in this molecule, all atoms are not coplanar.
So, the correct answer is option B.
Note- Coplanar means atoms or groups of atoms that lie on the same plane. Like biphenyl, it has both benzene rings on the same plane. When all atoms of a compound are in the same plane are called coplanar compounds. Co planarity in organic compounds is seen in unsaturated molecules.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




