
Increasing order of atomic radii
Li, Na, K, Rb, Cs:
(A) Li < Na < K < Rb < Cs
(B) Li < Na < K < Cs < Rb
(C) Li < K < Na < Rb < Cs
(D) None of these
Answer
584.4k+ views
Hint: In the periodic table, the elements of any group shows the continual behaviour within the group with respect to any property or trends applied to the same.
Complete step by step solution:
Let us first understand what do we mean by atomic radius and further we will move towards the trends in the modern periodic table.
Atomic radius- Atomic radius of a chemical element is basically the size of its atom. Typically, it is stated as the total distance from an atom’s nucleus to the outermost orbital of an electron.
The atomic radius of an atom is measured by X-ray or other spectroscopy methods.
The atomic radii of elements vary in the periodic table in a fixed pattern.
Atomic radius trends-
Period- Atomic size gradually decreases from left to right across the period of elements. This is because, within the period electrons are added in the same shell. However, an equal number of protons are added to the nucleus so that there is balance of the charges in an atom.
This results in greater nuclear attraction i.e. the nucleus attracts the electrons more strongly pulling the atom’s shell closer to the nucleus. Thus, the atomic radius decreases across the period.
Group- Down the group, atomic radius increases. This is because there are more energy levels and therefore a greater distance between protons and electrons of the same atom.
In addition, there is an electron shielding effect which decreases the attraction between the positively charged nucleus and negatively charged electrons. Thus, the electrons go farther away from the nucleus.
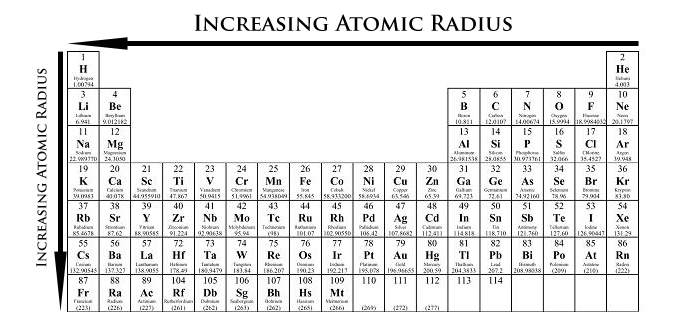
The picture here shows the trend in accordance with the atomic radius in the modern periodic table.
Illustration-
Here, we have five elements from Group 1 (IA, Alkali metals). Each one have been described below for better understanding;
(i) Lithium-
Atomic number- 3
Symbol- Li
Electronic configuration- 2, 1
Energy levels (electron shells)- 2
Atomic radius (pm)- 134
(ii) Sodium-
Atomic number- 11
Symbol- Na
Electronic configuration- 2, 8, 1
Energy levels (electron shells)- 3
Atomic radius (pm)- 154
(iii) Potassium-
Atomic number- 19
Symbol- K
Electronic configuration- 2, 8, 8, 1
Energy levels (electron shells)- 4
Atomic radius (pm3)- 196
(iv) Rubidium-
Atomic number- 37
Symbol- Rb
Electronic configuration- 2, 8, 18, 8, 1
Energy levels (electron shells)- 5
Atomic radius (pm)- 211
(v) Caesium-
Atomic number- 55
Symbol- Cs
Electronic configuration- 2, 8, 18, 18, 8, 1
Energy levels (electron shells)- 6
Atomic radius (pm)- 225
This shows us that lithium has the smallest atomic radius and caesium has the largest atomic radius within the given elements of a group.
Thus, the correct trend can be shown by option (A) Li < Na < K < Rb < Cs.
Note: The trend will anyhow be present for the groups and periods in the periodic table, do not get confused due to the option (D). In short, the atomic radius increases or decreases based on the effective nuclear charge.
Complete step by step solution:
Let us first understand what do we mean by atomic radius and further we will move towards the trends in the modern periodic table.
Atomic radius- Atomic radius of a chemical element is basically the size of its atom. Typically, it is stated as the total distance from an atom’s nucleus to the outermost orbital of an electron.
The atomic radius of an atom is measured by X-ray or other spectroscopy methods.
The atomic radii of elements vary in the periodic table in a fixed pattern.
Atomic radius trends-
Period- Atomic size gradually decreases from left to right across the period of elements. This is because, within the period electrons are added in the same shell. However, an equal number of protons are added to the nucleus so that there is balance of the charges in an atom.
This results in greater nuclear attraction i.e. the nucleus attracts the electrons more strongly pulling the atom’s shell closer to the nucleus. Thus, the atomic radius decreases across the period.
Group- Down the group, atomic radius increases. This is because there are more energy levels and therefore a greater distance between protons and electrons of the same atom.
In addition, there is an electron shielding effect which decreases the attraction between the positively charged nucleus and negatively charged electrons. Thus, the electrons go farther away from the nucleus.

The picture here shows the trend in accordance with the atomic radius in the modern periodic table.
Illustration-
Here, we have five elements from Group 1 (IA, Alkali metals). Each one have been described below for better understanding;
(i) Lithium-
Atomic number- 3
Symbol- Li
Electronic configuration- 2, 1
Energy levels (electron shells)- 2
Atomic radius (pm)- 134
(ii) Sodium-
Atomic number- 11
Symbol- Na
Electronic configuration- 2, 8, 1
Energy levels (electron shells)- 3
Atomic radius (pm)- 154
(iii) Potassium-
Atomic number- 19
Symbol- K
Electronic configuration- 2, 8, 8, 1
Energy levels (electron shells)- 4
Atomic radius (pm3)- 196
(iv) Rubidium-
Atomic number- 37
Symbol- Rb
Electronic configuration- 2, 8, 18, 8, 1
Energy levels (electron shells)- 5
Atomic radius (pm)- 211
(v) Caesium-
Atomic number- 55
Symbol- Cs
Electronic configuration- 2, 8, 18, 18, 8, 1
Energy levels (electron shells)- 6
Atomic radius (pm)- 225
This shows us that lithium has the smallest atomic radius and caesium has the largest atomic radius within the given elements of a group.
Thus, the correct trend can be shown by option (A) Li < Na < K < Rb < Cs.
Note: The trend will anyhow be present for the groups and periods in the periodic table, do not get confused due to the option (D). In short, the atomic radius increases or decreases based on the effective nuclear charge.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




