
Industrially, ${H_2}{O_2}$ is prepared by the auto-oxidation of 2-alkyl anthraquinone.
A. True
B. False
Answer
578.1k+ views
Hint: Auto-oxidation is any oxidation that occurs in the presence of oxygen. It produces hydro peroxides and cyclic organic peroxides.
Complete step by step answer:
Alkyl form of anthraquinone is 2-Ethylanthraquinols. It is an organic compound that produces anthraquinone. It is a pale yellow solid which is used in the industrial production of Hydrogen Peroxide (${H_2}{O_2}$).
When 2-alkyl anthraquinone undergoes oxidation it forms 2-alkyl anthraquinone along with hydrogen peroxide (${H_2}{O_2}$). The reaction is as given below.
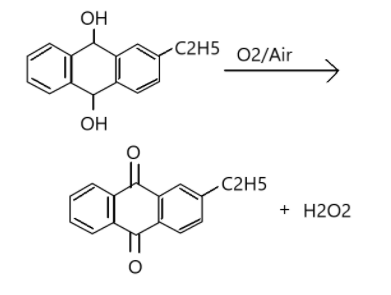
Therefore, the correct option is A.
Additional Information:
Anthraquinone which is also known as anthracenedione or dioxo anthracene is an aromatic organic compound with formula ${C_{14}}{H_8}{O_2}$ . Its isomers include various quinone derivatives. The term anthraquinone refers to the isomer 9,10-anthraquinone where the keto groups are located in the central ring. It is a building block of many dyes and is used in bleaching pulp for papermaking. It is a yellow, highly crystalline solid, poorly soluble in water but is soluble in hot organic solvents. It is almost completely insoluble in ethanol near room temperature but $2.25g$ will dissolve in $100g$ of boiling ethanol. It is found in nature as the rare mineral halite.
Hydrogen peroxide is a chemical compound with the formula ${H_2}{O_2}$ . In its pure form, it is a very pale blue liquid which is more viscous than water. It is the simplest peroxide which is used as an oxidizer, bleaching agent and antiseptic. Concentrated hydrogen peroxide or high-test peroxide is a reactive oxygen species and has been used as a propellant in rocketry. It is produced industrially by the anthraquinone process which involves using 2-alkyl-9,10-anthraquinones for hydrogenation. 2-ethylanthraquinone is common because of its high selectivity.
Note:
Auto oxidation is important because it is a useful reaction for converting compounds to oxygenated derivatives. Also, it occurs in situations where it is not desired. Auto oxidation of 2-Ethylanthraquinone is the main process for the formation of Hydrogen Peroxide.
Complete step by step answer:
Alkyl form of anthraquinone is 2-Ethylanthraquinols. It is an organic compound that produces anthraquinone. It is a pale yellow solid which is used in the industrial production of Hydrogen Peroxide (${H_2}{O_2}$).
When 2-alkyl anthraquinone undergoes oxidation it forms 2-alkyl anthraquinone along with hydrogen peroxide (${H_2}{O_2}$). The reaction is as given below.

Therefore, the correct option is A.
Additional Information:
Anthraquinone which is also known as anthracenedione or dioxo anthracene is an aromatic organic compound with formula ${C_{14}}{H_8}{O_2}$ . Its isomers include various quinone derivatives. The term anthraquinone refers to the isomer 9,10-anthraquinone where the keto groups are located in the central ring. It is a building block of many dyes and is used in bleaching pulp for papermaking. It is a yellow, highly crystalline solid, poorly soluble in water but is soluble in hot organic solvents. It is almost completely insoluble in ethanol near room temperature but $2.25g$ will dissolve in $100g$ of boiling ethanol. It is found in nature as the rare mineral halite.
Hydrogen peroxide is a chemical compound with the formula ${H_2}{O_2}$ . In its pure form, it is a very pale blue liquid which is more viscous than water. It is the simplest peroxide which is used as an oxidizer, bleaching agent and antiseptic. Concentrated hydrogen peroxide or high-test peroxide is a reactive oxygen species and has been used as a propellant in rocketry. It is produced industrially by the anthraquinone process which involves using 2-alkyl-9,10-anthraquinones for hydrogenation. 2-ethylanthraquinone is common because of its high selectivity.
Note:
Auto oxidation is important because it is a useful reaction for converting compounds to oxygenated derivatives. Also, it occurs in situations where it is not desired. Auto oxidation of 2-Ethylanthraquinone is the main process for the formation of Hydrogen Peroxide.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




