
What intermolecular forces are present in \[C{H_3}OH\] ?
Answer
533.7k+ views
Hint: Intermolecular forces refer to those forces that mediate interaction between the molecules and they include forces of attraction and repulsion which are supposed to act between the atoms or other neighbouring particles like atoms or ions. Different types of intermolecular forces include ionic bonds, Vander Waals dipole-dipole interaction, hydrogen bonding and Vander Waals dispersion forces.
Complete step by step answer:
The given compound methanol i.e. \[C{H_3}OH\] is polar in nature due to the presence of hydroxyl groups. In alcohols, the hydrogen bonds are formed owing to the existence of covalent bonds between oxygen atom and hydrogen atom in the hydroxyl group ( \[O--H\] ). Oxygen is highly electronegative and thus, attracts the electrons towards itself in \[O--H\] bonds. As the proton in the hydrogen atom slightly screens the action of the oxygen which pulls the electrons away from hydrogen, it results into a net positive charge over the hydrogen atom. As a result, there is a net negative charge on the oxygen atom which creates an imbalance of charge over the hydroxyl group. Thus, \[C{H_3}OH\] can be represented as:
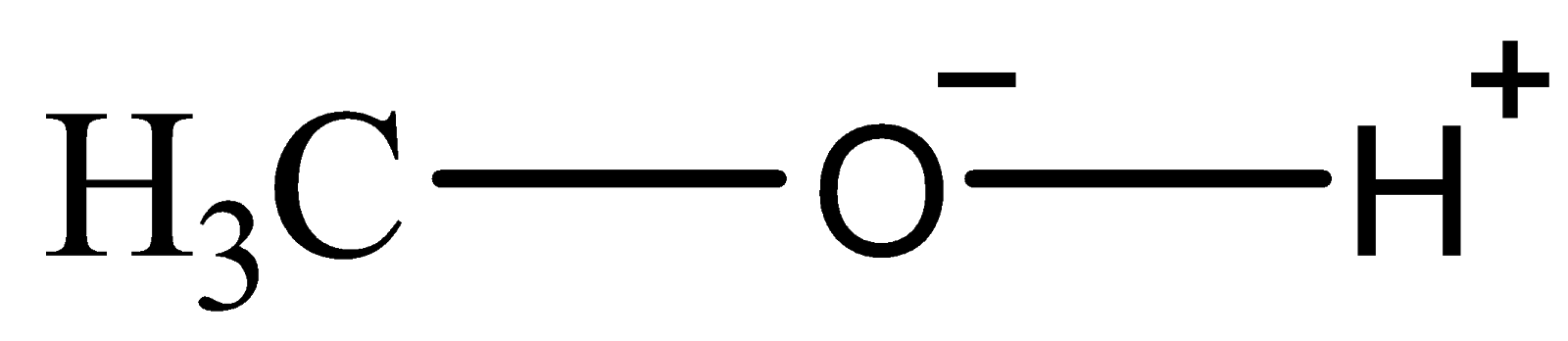
Thus, the overall hydroxyl group is considered to be polar (similar to magnet) as it possesses two opposite charges on each end. The net positive hydrogen atom can attract the negative electron clouds readily from the oxygen atom placed adjacent to it. Unlike Van der Waals’ forces, hydrogen bond involves a permanent imbalance of charges and hence, results in permanent dipole attractions.
The diagram below demonstrates hydrogen bonding in \[C{H_3}OH\] molecules.
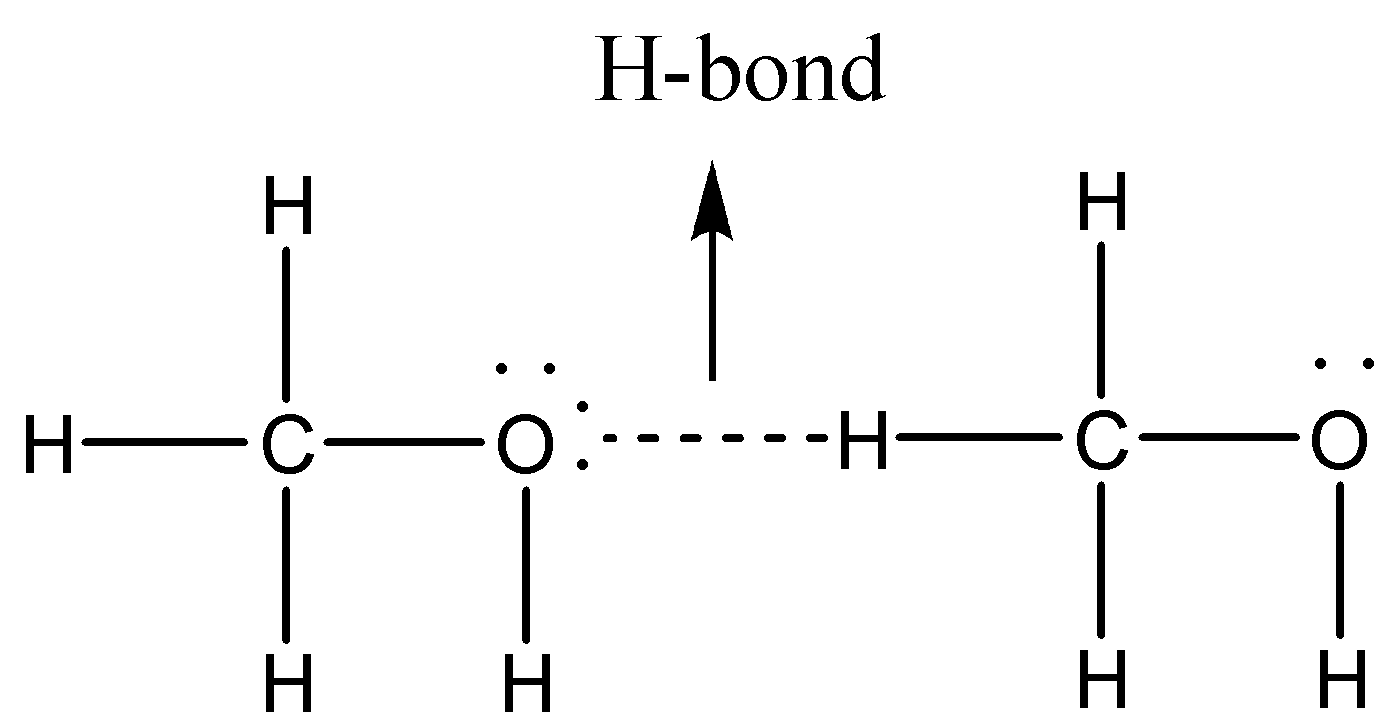
Now, we will discuss other intermolecular forces present in \[C{H_3}OH\] . As \[C{H_3}OH\] is polar in nature, it possesses a non-zero dipole. We know that oxygen atom is more electronegative in comparison to carbon atom and also, \[C{H_3}\] is considered to be a good electron donating group, so there will be a partial positive charge on carbon while partial negative charge on oxygen (i.e, a dipole), leading to dipole-dipole interactions as shown below:
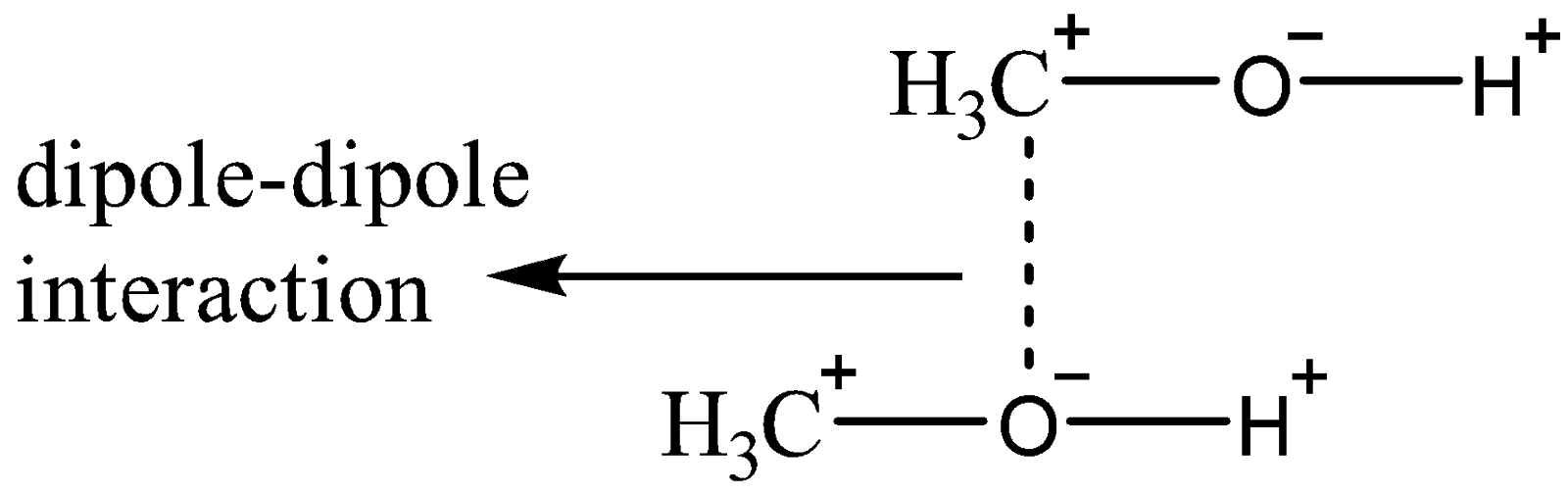
\[C{H_3}OH\] also experiences Van Der Waals dispersion forces though of very low magnitude. As we already know that when atoms or molecules are polarizable to a certain degree, some interactions occur from them when they are brought together as electrons are pushed about.
As a result, intermolecular forces such as hydrogen bonding, dipole-dipole interaction and Vander Waals dispersion forces are present in \[C{H_3}OH\] .
Note: Hydrogen bonding in alcohols make them soluble in water. Alcohols with a smaller hydrocarbon chain are highly soluble in water while alcohols having a higher hydrocarbon chain are less soluble in water owing to increasing hydrophobicity of the alkyl chain.
Complete step by step answer:
The given compound methanol i.e. \[C{H_3}OH\] is polar in nature due to the presence of hydroxyl groups. In alcohols, the hydrogen bonds are formed owing to the existence of covalent bonds between oxygen atom and hydrogen atom in the hydroxyl group ( \[O--H\] ). Oxygen is highly electronegative and thus, attracts the electrons towards itself in \[O--H\] bonds. As the proton in the hydrogen atom slightly screens the action of the oxygen which pulls the electrons away from hydrogen, it results into a net positive charge over the hydrogen atom. As a result, there is a net negative charge on the oxygen atom which creates an imbalance of charge over the hydroxyl group. Thus, \[C{H_3}OH\] can be represented as:
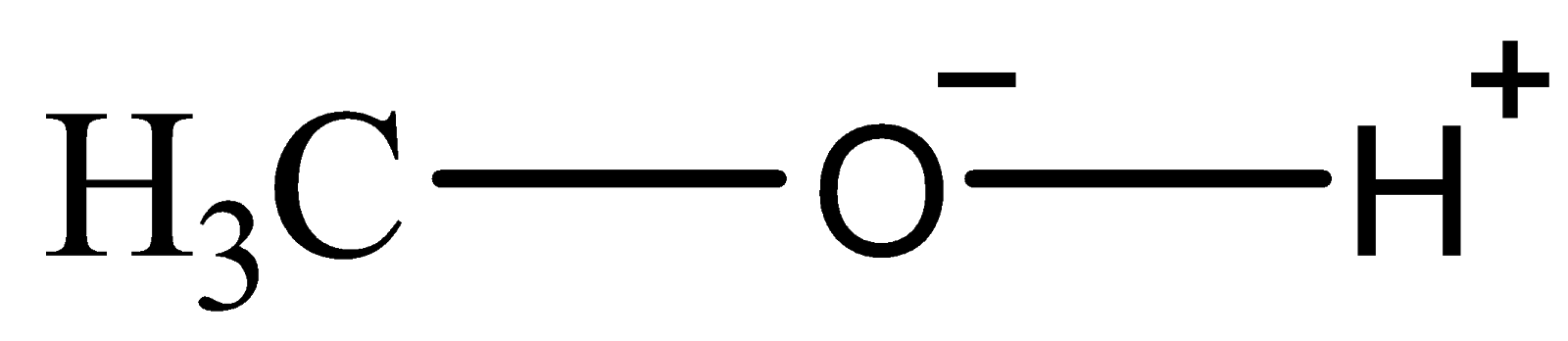
Thus, the overall hydroxyl group is considered to be polar (similar to magnet) as it possesses two opposite charges on each end. The net positive hydrogen atom can attract the negative electron clouds readily from the oxygen atom placed adjacent to it. Unlike Van der Waals’ forces, hydrogen bond involves a permanent imbalance of charges and hence, results in permanent dipole attractions.
The diagram below demonstrates hydrogen bonding in \[C{H_3}OH\] molecules.

Now, we will discuss other intermolecular forces present in \[C{H_3}OH\] . As \[C{H_3}OH\] is polar in nature, it possesses a non-zero dipole. We know that oxygen atom is more electronegative in comparison to carbon atom and also, \[C{H_3}\] is considered to be a good electron donating group, so there will be a partial positive charge on carbon while partial negative charge on oxygen (i.e, a dipole), leading to dipole-dipole interactions as shown below:

\[C{H_3}OH\] also experiences Van Der Waals dispersion forces though of very low magnitude. As we already know that when atoms or molecules are polarizable to a certain degree, some interactions occur from them when they are brought together as electrons are pushed about.
As a result, intermolecular forces such as hydrogen bonding, dipole-dipole interaction and Vander Waals dispersion forces are present in \[C{H_3}OH\] .
Note: Hydrogen bonding in alcohols make them soluble in water. Alcohols with a smaller hydrocarbon chain are highly soluble in water while alcohols having a higher hydrocarbon chain are less soluble in water owing to increasing hydrophobicity of the alkyl chain.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




