
What is the ionic bond formation of calcium and sulphur?
Answer
524.4k+ views
Hint : Factors which favour the formation of ionic bond are ionization enthalpy, electron gain enthalpy and lattice enthalpy. The energy change occurs due to formation of anion and cation, also in packing of ions of opposite charge.
Complete Step By Step Answer:
Ionic bond is formed by the transference of one or more electrons from one atom to the other. This type of bond usually comes into existence between a metal and a nonmetal atom.
The formation of ionic bond primarily depends upon:
\[1.\] The ease of formation of positive and negative ions from their respective neutral atoms.
\[2.\] The arrangement of the positive and negative ions in the solid, that is, the lattice of the crystalline compound.
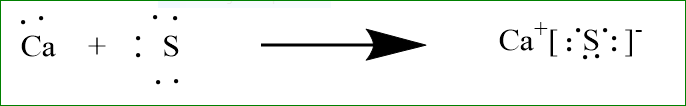
Let us study the formation of ionic bond formation of calcium and sulphur.
Sulphur has six electrons in its valence shell and it requires two electrons to attain stable gas configuration of argon. Similarly, calcium has two electrons in its valence shell and it loses two electrons to attain stable gas configuration of krypton. So, calcium transfers its valence electrons to sulphur and both form\[N{a^ + }{\text{ and }}C{l^ - }\]respectively. The electrostatic force of attraction holds these oppositely charged ions together. The above changes can be represented as:
Note :
The number of electrons which an atom loses or gains while forming an ionic bond is called its Electrovalency. The atom which loses electrons is called electropositive atom while the atom which gains an electron is called electronegative atom. Ions has a uniform field of influence around it, hence, the ionic bond is non non-directional in nature.
Complete Step By Step Answer:
Ionic bond is formed by the transference of one or more electrons from one atom to the other. This type of bond usually comes into existence between a metal and a nonmetal atom.
The formation of ionic bond primarily depends upon:
\[1.\] The ease of formation of positive and negative ions from their respective neutral atoms.
\[2.\] The arrangement of the positive and negative ions in the solid, that is, the lattice of the crystalline compound.
Let us study the formation of ionic bond formation of calcium and sulphur.
Sulphur has six electrons in its valence shell and it requires two electrons to attain stable gas configuration of argon. Similarly, calcium has two electrons in its valence shell and it loses two electrons to attain stable gas configuration of krypton. So, calcium transfers its valence electrons to sulphur and both form\[N{a^ + }{\text{ and }}C{l^ - }\]respectively. The electrostatic force of attraction holds these oppositely charged ions together. The above changes can be represented as:

Note :
The number of electrons which an atom loses or gains while forming an ionic bond is called its Electrovalency. The atom which loses electrons is called electropositive atom while the atom which gains an electron is called electronegative atom. Ions has a uniform field of influence around it, hence, the ionic bond is non non-directional in nature.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




