
Is \[2 - \] hydroxy propanoic acid optically active?
Answer
498.3k+ views
Hint: Optical activity of a compound is defined as the ability of a substance or compound to rotate the plane polarized light. When the plane polarized light passes through the solution of the substance, it can rotate the plane polarized light either in the clockwise direction or in the anti-clockwise direction.
Complete answer:
Optical activity of the compound is the function of the presence or the absence of the chiral atom in it.
So, the optically active compound can be in the dextrorotatory form and the Laevorotatory form.
So, let’s see the structure of the \[2 - \] hydroxy propanoic acid.
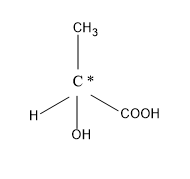
As we can see from the above figure that the \[2 - \] hydroxyl propanoic acid has a centre chiral atom or carbon, it has four different groups satisfying its valence. The four different groups bonded to the chiral centre are the hydrogen, the methyl group, the carboxylic group and the hydroxyl group.
So, we can say it is very clear from the above discussion that the \[2 - \] hydroxyl propanoic acid is an optically active compound as it has a chiral centre in it.
So the answer to our question is –: yes \[2 - \] hydroxy propanoic acid is optically active.
Note:
So, let’s see what is the chiral atom – so chiral atom is the atom which has all four different groups bonded to it. Only those substances are optically active, which have a chiral centre. Generally a chiral centre is denoted by the ‘asterisk sign’. This is an important aspect in determining structure.
Complete answer:
Optical activity of the compound is the function of the presence or the absence of the chiral atom in it.
So, the optically active compound can be in the dextrorotatory form and the Laevorotatory form.
So, let’s see the structure of the \[2 - \] hydroxy propanoic acid.

As we can see from the above figure that the \[2 - \] hydroxyl propanoic acid has a centre chiral atom or carbon, it has four different groups satisfying its valence. The four different groups bonded to the chiral centre are the hydrogen, the methyl group, the carboxylic group and the hydroxyl group.
So, we can say it is very clear from the above discussion that the \[2 - \] hydroxyl propanoic acid is an optically active compound as it has a chiral centre in it.
So the answer to our question is –: yes \[2 - \] hydroxy propanoic acid is optically active.
Note:
So, let’s see what is the chiral atom – so chiral atom is the atom which has all four different groups bonded to it. Only those substances are optically active, which have a chiral centre. Generally a chiral centre is denoted by the ‘asterisk sign’. This is an important aspect in determining structure.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




